Polymer Photoelectrodes for Solar Fuel Production: Progress and Challenges
- PMID: 35699661
- PMCID: PMC9284560
- DOI: 10.1021/acs.chemrev.1c00971
Polymer Photoelectrodes for Solar Fuel Production: Progress and Challenges
Abstract
Converting solar energy to fuels has attracted substantial interest over the past decades because it has the potential to sustainably meet the increasing global energy demand. However, achieving this potential requires significant technological advances. Polymer photoelectrodes are composed of earth-abundant elements, e.g. carbon, nitrogen, oxygen, hydrogen, which promise to be more economically sustainable than their inorganic counterparts. Furthermore, the electronic structure of polymer photoelectrodes can be more easily tuned to fit the solar spectrum than inorganic counterparts, promising a feasible practical application. As a fast-moving area, in particular, over the past ten years, we have witnessed an explosion of reports on polymer materials, including photoelectrodes, cocatalysts, device architectures, and fundamental understanding experimentally and theoretically, all of which have been detailed in this review. Furthermore, the prospects of this field are discussed to highlight the future development of polymer photoelectrodes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures











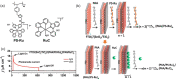
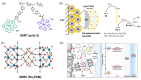













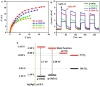





References
-
- Hall D. O.; Rao K.. Photosynthesis; Cambridge University Press, 1999.
-
- Ahmad T.; Zhang D. A Critical Review of Comparative Global Historical Energy Consumption and Future Demand: The Story Told so Far. Energy Rep. 2020, 6, 1973–1991. 10.1016/j.egyr.2020.07.020. - DOI
-
- Saunders N.; Chapman S.. Fossil Fuel; Raintree, 2005.
-
- Global Energy Transformation: A Roadmap to 2050; IRENA, 2019.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

