Macrophage-NLRP3 Activation Promotes Right Ventricle Failure in Pulmonary Arterial Hypertension
- PMID: 35699679
- PMCID: PMC9716901
- DOI: 10.1164/rccm.202110-2274OC
Macrophage-NLRP3 Activation Promotes Right Ventricle Failure in Pulmonary Arterial Hypertension
Abstract
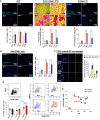
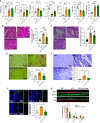
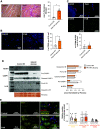
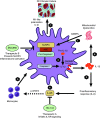
Rationale: Pulmonary arterial hypertension (PAH) often results in death from right ventricular failure (RVF). NLRP3 (nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3)-macrophage activation may promote RVF in PAH. Objectives: Evaluating the contribution of the NLRP3 inflammasome in RV macrophages to PAH RVF. Methods: Rats with decompensated RV hypertrophy (monocrotaline [MCT] and Sugen-5416 hypoxia [SuHx]) were compared with compensated RV hypertrophy rats (pulmonary artery banding). Echocardiography and right heart catheterization were performed. Macrophages, atrial natriuretic peptides, and fibrosis were evaluated by microscopy or flow cytometry. NLRP3 inflammasome activation and cardiotoxicity were confirmed by immunoblot and in vitro strategies. MCT rats were treated with SC-144 (a GP130 antagonist) or MCC950 (an NLRP3 inhibitor). Macrophage-NLRP3 activity was evaluated in patients with PAH RVF. Measurements and Main Results: Macrophages, fibrosis, and atrial natriuretic peptides were increased in MCT and SuHx RVs but not in left ventricles or pulmonary artery banding rats. Although MCT RV macrophages were inflammatory, lung macrophages were antiinflammatory. CCR2+ macrophages (monocyte-derived) were increased in MCT and SuHx RVs and highly expressed NLRP3. The macrophage-NLRP3 pathway was upregulated in patients with PAH with decompensated RVs. Cultured MCT monocytes showed NLRP3 activation, and in coculture experiments resulted in cardiomyocyte mitochondrial damage, which MCC950 prevented. In vivo, MCC950 reduced NLRP3 activation and regressed pulmonary vascular disease and RVF. SC-144 reduced RV macrophages and NLRP3 content, prevented STAT3 (signal transducer and activator of transcription 3) activation, and improved RV function without regressing pulmonary vascular disease. Conclusions: NLRP3-macrophage activation occurs in the decompensated RV in preclinical PAH models and patients with PAH. Inhibiting GP130 or NLRP3 signaling improves RV function. The concept that PAH RVF results from RV inflammation rather than solely from elevated RV afterload suggests a new therapeutic paradigm.
Keywords: CCR2; IL-1β; MCC950; SC-144; mitochondrial fission.
Figures



Comment in
-
Misbehaving Guests in the Right Ventricle: Macrophage-NLRP3 Activation in Pulmonary Hypertension.Am J Respir Crit Care Med. 2022 Sep 1;206(5):532-534. doi: 10.1164/rccm.202205-0977ED. Am J Respir Crit Care Med. 2022. PMID: 35704289 Free PMC article. No abstract available.
References
-
- Vonk-Noordegraaf A, Haddad F, Chin KM, Forfia PR, Kawut SM, Lumens J, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol . 2013;62:D22–D33. - PubMed
-
- Tian L, Wu D, Dasgupta A, Chen KH, Mewburn J, Potus F, et al. Epigenetic metabolic reprogramming of right ventricular fibroblasts in pulmonary arterial hypertension: a pyruvate dehydrogenase kinase-dependent shift in mitochondrial metabolism promotes right ventricular fibrosis. Circ Res . 2020;126:1723–1745. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

