Uncertainty estimation strategies for quantitative non-targeted analysis
- PMID: 35699740
- PMCID: PMC9465984
- DOI: 10.1007/s00216-022-04118-z
Uncertainty estimation strategies for quantitative non-targeted analysis
Abstract
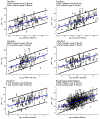
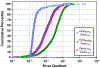
Non-targeted analysis (NTA) methods are widely used for chemical discovery but seldom employed for quantitation due to a lack of robust methods to estimate chemical concentrations with confidence limits. Herein, we present and evaluate new statistical methods for quantitative NTA (qNTA) using high-resolution mass spectrometry (HRMS) data from EPA's Non-Targeted Analysis Collaborative Trial (ENTACT). Experimental intensities of ENTACT analytes were observed at multiple concentrations using a semi-automated NTA workflow. Chemical concentrations and corresponding confidence limits were first estimated using traditional calibration curves. Two qNTA estimation methods were then implemented using experimental response factor (RF) data (where RF = intensity/concentration). The bounded response factor method used a non-parametric bootstrap procedure to estimate select quantiles of training set RF distributions. Quantile estimates then were applied to test set HRMS intensities to inversely estimate concentrations with confidence limits. The ionization efficiency estimation method restricted the distribution of likely RFs for each analyte using ionization efficiency predictions. Given the intended future use for chemical risk characterization, predicted upper confidence limits (protective values) were compared to known chemical concentrations. Using traditional calibration curves, 95% of upper confidence limits were within ~tenfold of the true concentrations. The error increased to ~60-fold (ESI+) and ~120-fold (ESI-) for the ionization efficiency estimation method and to ~150-fold (ESI+) and ~130-fold (ESI-) for the bounded response factor method. This work demonstrates successful implementation of confidence limit estimation strategies to support qNTA studies and marks a crucial step towards translating NTA data in a risk-based context.
Keywords: ENTACT; Exposure; HRMS; NTA; Quantitative; Uncertainty.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Figures
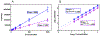
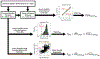
References
-
- Egeghy PP, Judson R, Gangwal S, Mosher S, Smith D, Vail J, et al. The exposure data landscape for manufactured chemicals. Sci Total Environ. 2012:414:159–66. - PubMed
-
- Weinberg N, Nelson D, Sellers K, Byrd J. Insights from TSCA reform: a case for identifying new emerging contaminants. Curr Pollut Rep. 2019;5(4):215–27.
-
- Risk assessment in the federal government: managing the process. National Research Council (US). Washington (DC): National Academies Press (US); 1983. - PubMed
-
- Postigo C, Andersson A, Harir M, Bastviken D, Gonsior M, Schmitt-Kopplin P, et al. Unraveling the chemodiversity of halogenated disinfection by-products formed during drinking water treatment using target and non-target screening tools. J Hazard Mater. 2021;401. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

