Effect of sample management on quantitative HIV-1 viral load measurement at Saint Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia
- PMID: 35700178
- PMCID: PMC9197020
- DOI: 10.1371/journal.pone.0269943
Effect of sample management on quantitative HIV-1 viral load measurement at Saint Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia
Abstract
Purpose: This study was meant to determine the effect of time to plasma separation, storage duration, freeze-thawing cycle and dilution proportion on the HIV-1 viral load level.
Methods: Experimental study design was employed by collecting 10mL whole blood samples into two EDTA tubes from 88 eligible HIV infected patients at St Paul's Hospital Millennium Medical College. The viral load test was done using Abbott m2000sp/rt analyzer. Data was entered into Microsoft excel and analyzed by SPSS version 20. Repeated measure analysis of variance was used to compare HIV RNA viral load mean difference between different time to plasma separation, storage, freeze-thawing cycles and dilution levels. Post-hoc analysis was employed to locate the place of significant differences. P value less than 0.05 was used to declare statistical significance while viral RNA level of 0.5 log copies/ml was used to determine clinical significance.
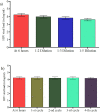
Results: There was significant HIV-1 RNA viral load log mean difference between plasma separation time at 6 hours (hrs) and 24hrs (p<0.001). There was also significant HIV-1 RNA viral load log mean difference between plasma tested within 6hrs and those stored at 2-8°C for 15 days (p = 0.006), and between plasma stored at 2-8°C for 6 days versus 15 days (p<0.001). There was significant log mean difference between plasma that was exposed to fourth cycle of freeze-thawing after storage at -20°C when compared with plasma tested within 6hrs (p = 0.013).
Conclusion: Plasma separated at 24hrs, stored at 2-8°C for 15 days or freeze-thawed for four cycles had significant effect on HIV viral load level. However, the differences were not clinically significant at a cut-off viral load level of 0.5 log copies/ml. Avoiding delays to plasma separation beyond 24 hrs, storing at 2-8°C for 15 days and freeze-thawing for no more than 4 cycles is recommended to improve the result quality.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- WHO HIV treatment and care, what’s new in treatment monitoring: viral load and CD4 testing, update July 2017 (www.who.int/hiv). Accessed on August 27/2019.
-
- WHO, Technical and operational considerations for implementing HIV viral load testing, World Health Organization 2014 (www.who.int/about/licensing//en/index.html). Accessed on February 03/2018.
-
- Doherty M, WHO guidelines on the use of CD4, Viral Load and EID tests for initiation and monitoring of ART, Treatment and Care Unit WHO,2013. Geneva (www.who.int/about/licensing//en/index.html). Accessed on February 03/2018.
-
- Christine C, Ginocchio W, Mark H, Kaplan M, Donald W, Joseph W, et al.. Effects of Specimen Collection, Processing, and Storage Conditions on Stability of Human Immunodeficiency Virus Type 1 RNA Levels in Plasma. J. Clin. Microbiol 1997; 35(11): 2886–2893. doi: 10.1128/jcm.35.11.2886-2893.1997 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical