Persistence of gastrointestinal symptoms in irritable bowel syndrome and ulcerative colitis: study protocol for a three-arm randomised controlled trial (SOMA.GUT-RCT)
- PMID: 35701050
- PMCID: PMC9198710
- DOI: 10.1136/bmjopen-2021-059529
Persistence of gastrointestinal symptoms in irritable bowel syndrome and ulcerative colitis: study protocol for a three-arm randomised controlled trial (SOMA.GUT-RCT)
Abstract
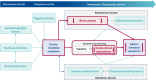
Introduction: Ulcerative colitis (UC) and irritable bowel syndrome (IBS) are distressing chronic diseases associated with abdominal pain and altered bowel habits of unknown aetiology. Results from previous studies indicate that, across both diseases, increased levels of illness-related anxiety and dysfunctional symptom expectations contribute to symptom persistence. Thus, comparing both disorders with regard to common and disease-specific factors in the persistence and modification of gastrointestinal symptoms seems justified. Our primary hypothesis is that persistent gastrointestinal symptoms in UC and IBS can be improved by modifying dysfunctional symptom expectations and illness-related anxiety using expectation management strategies.
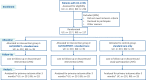
Methods and analysis: To assess the extent to which persistent somatic symptoms are modifiable in adult patients with UC and IBS, we will conduct an observer-blinded, three-arm randomised controlled trial. A total of 117 patients with UC and 117 patients with IBS will be randomised into three groups of equal size: targeted expectation management aiming to reduce illness-related anxiety and dysfunctional symptom expectations in addition to standard care (SC, intervention 1), non-specific supportive treatment in addition to SC (intervention 2) or SC only (control). Both active intervention groups will comprise three individual online consultation sessions and a booster session after 3 months. The primary outcome is baseline to postinterventional change in gastrointestinal symptom severity.
Ethics and dissemination: The study was approved by the Ethics Committee of the Hamburg Medical Association (2020-10198-BO-ff). The study will shed light onto the efficacy and mechanisms of action of a targeted expectation management intervention for persistent gastrointestinal symptoms in patients with UC and IBS. Furthermore, the detailed analysis of the complex biopsychosocial mechanisms will allow the further advancement of aetiological models and according evidence-based intervention strategies.
Trial registration number: ISRCTN30800023.
Keywords: Functional bowel disorders; Gastroenterology; Inflammatory bowel disease; MENTAL HEALTH.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Henriksen M, Høivik ML, Jelsness-Jørgensen L-P, et al. . Irritable bowel-like symptoms in ulcerative colitis are as common in patients in deep remission as in inflammation: Results from a population-based study [the IBSEN study]. Journal of Crohn’s colitis 2018;12:389–93. 10.1093/ecco-jcc/jjx152 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
