Polybasic Patches in Both C2 Domains of Synaptotagmin-1 Are Required for Evoked Neurotransmitter Release
- PMID: 35701163
- PMCID: PMC9337609
- DOI: 10.1523/JNEUROSCI.1385-21.2022
Polybasic Patches in Both C2 Domains of Synaptotagmin-1 Are Required for Evoked Neurotransmitter Release
Abstract
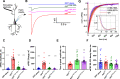
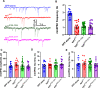
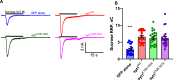
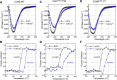
Synaptotagmin-1 (Syt1) is a vesicular calcium sensor required for synchronous neurotransmitter release, composed of a single-pass transmembrane domain linked to two C2 domains (C2A and C2B) that bind calcium, acidic lipids, and SNARE proteins that drive fusion of the synaptic vesicle with the plasma membrane. Despite its essential role, how Syt1 couples calcium entry to synchronous release is poorly understood. Calcium binding to C2B is critical for synchronous release, and C2B additionally binds the SNARE complex. The C2A domain is also required for Syt1 function, but it is not clear why. Here, we asked what critical feature of C2A may be responsible for its functional role and compared this to the analogous feature in C2B. We focused on highly conserved poly-lysine patches located on the sides of C2A (K189-192) and C2B (K324-327). We tested effects of charge-neutralization mutations in either region (Syt1K189-192A and Syt1K326-327A) side by side to determine their relative contributions to Syt1 function in cultured cortical neurons from mice of either sex and in single-molecule experiments. Combining electrophysiological recordings and optical tweezers measurements to probe dynamic single C2 domain-membrane interactions, we show that both C2A and C2B polybasic patches contribute to membrane binding, and both are required for evoked release. The size of the readily releasable vesicle pool and the rate of spontaneous release were unaffected, so both patches are likely required specifically for synchronization of release. We suggest these patches contribute to cooperative membrane binding, increasing the overall affinity of Syt1 for negatively charged membranes and facilitating evoked release.SIGNIFICANCE STATEMENT Synaptotagmin-1 is a vesicular calcium sensor required for synchronous neurotransmitter release. Its tandem cytosolic C2 domains (C2A and C2B) bind calcium, acidic lipids, and SNARE proteins that drive fusion of the synaptic vesicle with the plasma membrane. How calcium binding to Synaptotagmin-1 leads to release and the relative contributions of the C2 domains are unclear. Combining electrophysiological recordings from cultured neurons and optical tweezers measurements of single C2 domain-membrane interactions, we show that conserved polybasic regions in both domains contribute to membrane binding cooperatively, and both are required for evoked release, likely by increasing the overall affinity of Synaptotagmin-1 for acidic membranes.
Keywords: calcium-triggered exocytosis; exocytosis; membrane–protein interactions; neurotransmitter release; synaptotagmin.
Copyright © 2022 the authors.
Figures



References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
