The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family
- PMID: 35701220
- PMCID: PMC9248827
- DOI: 10.1101/cshperspect.a041046
The Mitochondrial Pathway of Apoptosis Part II: The BCL-2 Protein Family
Figures
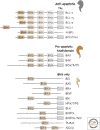



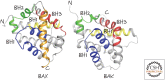

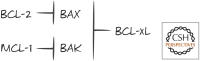
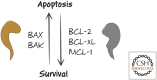

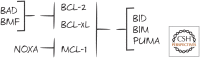

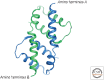
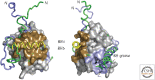



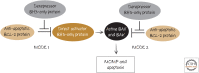

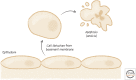
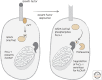
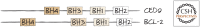




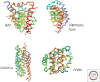

References
-
- Green DR. 2022. a. Cell death and cancer. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041103 - DOI
FIGURE CREDITS
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
