Disruption of spatiotemporal clustering in dengue cases by wMel Wolbachia in Yogyakarta, Indonesia
- PMID: 35701454
- PMCID: PMC9198086
- DOI: 10.1038/s41598-022-13749-2
Disruption of spatiotemporal clustering in dengue cases by wMel Wolbachia in Yogyakarta, Indonesia
Abstract
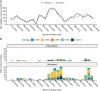
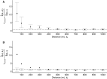
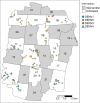
Dengue exhibits focal clustering in households and neighborhoods, driven by local mosquito population dynamics, human population immunity, and fine scale human and mosquito movement. We tested the hypothesis that spatiotemporal clustering of homotypic dengue cases is disrupted by introduction of the arbovirus-blocking bacterium Wolbachia (wMel-strain) into the Aedes aegypti mosquito population. We analysed 318 serotyped and geolocated dengue cases (and 5921 test-negative controls) from a randomized controlled trial in Yogyakarta, Indonesia of wMel deployments. We find evidence of spatial clustering up to 300 m among the 265 dengue cases (3083 controls) in the untreated trial arm. Participant pairs enrolled within 30 days and 50 m had a 4.7-fold increase (compared to 95% CI on permutation-based null distribution: 0.1, 1.2) in the odds of being homotypic (i.e. potentially transmission-related) as compared to pairs occurring at any distance. In contrast, we find no evidence of spatiotemporal clustering among the 53 dengue cases (2838 controls) resident in the wMel-treated arm. Introgression of wMel Wolbachia into Aedes aegypti mosquito populations interrupts focal dengue virus transmission leading to reduced case incidence; the true intervention effect may be greater than the 77% efficacy measured in the primary analysis of the Yogyakarta trial.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

