Spatial heterogeneity in DNA methylation and chromosomal alterations in diffuse gliomas and meningiomas
- PMID: 35701666
- PMCID: PMC9596370
- DOI: 10.1038/s41379-022-01113-8
Spatial heterogeneity in DNA methylation and chromosomal alterations in diffuse gliomas and meningiomas
Abstract
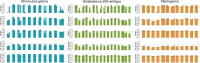
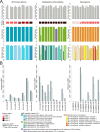
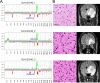
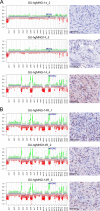
Adult-type diffuse gliomas and meningiomas are the most common primary intracranial tumors of the central nervous system. DNA methylation profiling is a novel diagnostic technique increasingly used also in the clinic. Although molecular heterogeneity is well described in these tumors, DNA methylation heterogeneity is less studied. We therefore investigated the intratumor genetic and epigenetic heterogeneity in diffuse gliomas and meningiomas, with focus on potential clinical implications. We further investigated tumor purity as a source for heterogeneity in the tumors. We analyzed genome-wide DNA methylation profiles generated from 126 spatially separated tumor biopsies from 39 diffuse gliomas and meningiomas. Moreover, we evaluated five methods for measurement of tumor purity and investigated intratumor heterogeneity by assessing DNA methylation-based classification, chromosomal copy number alterations and molecular markers. Our results demonstrated homogeneous methylation-based classification of IDH-mutant gliomas and further corroborates subtype heterogeneity in glioblastoma IDH-wildtype and high-grade meningioma patients after excluding samples with low tumor purity. We detected a large number of differentially methylated CpG sites within diffuse gliomas and meningiomas, particularly in tumors of higher grades. The presence of CDKN2A/B homozygous deletion differed in one out of two patients with IDH-mutant astrocytomas, CNS WHO grade 4. We conclude that diffuse gliomas and high-grade meningiomas are characterized by intratumor heterogeneity, which should be considered in clinical diagnostics and in the assessment of methylation-based and molecular markers.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Longitudinal DNA methylation analysis of adult-type IDH-mutant gliomas.Acta Neuropathol Commun. 2023 Feb 4;11(1):23. doi: 10.1186/s40478-023-01520-1. Acta Neuropathol Commun. 2023. PMID: 36739454 Free PMC article.
-
CDKN2A homozygous deletion is a strong adverse prognosis factor in diffuse malignant IDH-mutant gliomas.Neuro Oncol. 2019 Dec 17;21(12):1519-1528. doi: 10.1093/neuonc/noz124. Neuro Oncol. 2019. PMID: 31832685 Free PMC article.
-
Spatial concordance of DNA methylation classification in diffuse glioma.Neuro Oncol. 2021 Dec 1;23(12):2054-2065. doi: 10.1093/neuonc/noab134. Neuro Oncol. 2021. PMID: 34049406 Free PMC article.
-
Beyond the World Health Organization classification of central nervous system tumors 2016: what are the new developments for gliomas from a clinician's perspective?Curr Opin Neurol. 2020 Dec;33(6):701-706. doi: 10.1097/WCO.0000000000000871. Curr Opin Neurol. 2020. PMID: 33177376 Review.
-
Integrated diagnostics of diffuse astrocytic and oligodendroglial tumors.Pathologe. 2019 Jun;40(Suppl 1):9-17. doi: 10.1007/s00292-019-0581-8. Pathologe. 2019. PMID: 31025086 Review. English.
Cited by
-
The clinical value of proneural, classical and mesenchymal protein signatures in WHO 2021 adult-type diffuse lower-grade gliomas.PLoS One. 2023 May 16;18(5):e0285732. doi: 10.1371/journal.pone.0285732. eCollection 2023. PLoS One. 2023. PMID: 37192181 Free PMC article.
-
Evaluation of DNA Methylation Array for Glioma Tumor Profiling and Description of a Novel Epi-Signature to Distinguish IDH1/IDH2 Mutant and Wild-Type Tumors.Genes (Basel). 2022 Nov 9;13(11):2075. doi: 10.3390/genes13112075. Genes (Basel). 2022. PMID: 36360312 Free PMC article.
-
Utility of genome-wide DNA methylation profiling for pediatric-type diffuse gliomas.Brain Tumor Pathol. 2023 Apr;40(2):56-65. doi: 10.1007/s10014-023-00457-6. Epub 2023 Apr 1. Brain Tumor Pathol. 2023. PMID: 37004583 Review.
-
Regulatory function of DNA methylation mediated lncRNAs in gastric cancer.Cancer Cell Int. 2022 Jul 9;22(1):227. doi: 10.1186/s12935-022-02648-1. Cancer Cell Int. 2022. PMID: 35810299 Free PMC article. Review.
-
Novel Human Meningioma Organoids Recapitulate the Aggressiveness of the Initiating Cell Subpopulations Identified by ScRNA-Seq.Adv Sci (Weinh). 2023 May;10(15):e2205525. doi: 10.1002/advs.202205525. Epub 2023 Mar 30. Adv Sci (Weinh). 2023. PMID: 36994665 Free PMC article.
References
-
- Dagogo-Jack I & Shaw A. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 15, 81–94 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

