High androgen concentrations in follicular fluid of polycystic ovary syndrome women
- PMID: 35701786
- PMCID: PMC9195430
- DOI: 10.1186/s12958-022-00959-6
High androgen concentrations in follicular fluid of polycystic ovary syndrome women
Abstract
Background: According to current definitions of Polycystic Ovary Syndrome (PCOS), hyperandrogenism is considered as a key element in the pathogenesis of this common endocrinopathy. However, until now, studies about ovarian androgen profile in women are very rare. Our aim was then to characterise the expression profile of the androgens in follicular fluid of 30 PCOS patients, and compare it to those of 47 Control women and 29 women with only polycystic ovary morphology on ultrasounds (ECHO group).
Methods: A retrospective, single-centre cohort study was performed. The intrafollicular concentrations of the key androgens were assessed and correlated with the intrafollicular levels of some adipokines of interest. Androgens were quantified by mass spectrophotometry combined with ultra-high-performance liquid chromatography, while adipokine concentrations were measured by ELISA assays.
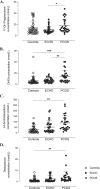
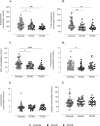
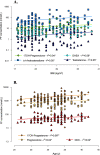
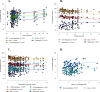
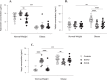
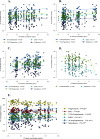
Results: In PCOS patients, the intrafollicular concentrations of the androgens synthesised by ovarian theca cells, i.e., 17OH-pregnenolone, dehydroepiandrosterone, Δ4-androstenedione and testosterone, were significantly higher than those of the androgens of adrenal origin, and positively correlated with the main PCOS clinical and biological features, as well as with the adipokines mostly expressed in the follicular fluid of PCOS patients, i.e. resistin, omentin, chemerin and apelin. Conversely, Control women showed the highest levels of 17OH-progesterone, deoxycorticosterone and 11-deoxycortisol. Confirming these results, apelin levels were negatively associated with pregnenolone and deoxycorticosterone concentrations, while visfatin levels, which were higher in the Control group, negatively correlated with the Δ4-androstenedione and testosterone ones.
Conclusions: PCOS is characterised by a selective increase in the intrafollicular levels of the androgens synthesised by theca cells, strengthening the hypothesis that ovarian hyperandrogenism plays a central role in its pathogenesis. Further, the significant correlation between the intrafollicular concentrations of the androgens and most of the adipokines of interest, including apelin, chemerin, resistin and omentin, confirms the existence of a close relationship between these two hormonal systems, which appear deeply involved in ovarian physiology and PCOS physiopathology.
Keywords: Adipokines; Androgens; Follicular fluid; Polycystic ovary syndrome (PCOS); Theca cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

