Effect of hyperthermic intraperitoneal chemotherapy in combination with cytoreductive surgery on the prognosis of patients with colorectal cancer peritoneal metastasis: a systematic review and meta-analysis
- PMID: 35701802
- PMCID: PMC9195265
- DOI: 10.1186/s12957-022-02666-3
Effect of hyperthermic intraperitoneal chemotherapy in combination with cytoreductive surgery on the prognosis of patients with colorectal cancer peritoneal metastasis: a systematic review and meta-analysis
Abstract
Background: Peritoneal metastasis often occurs in patients with colorectal cancer peritoneal metastasis, and the prognosis is poor. A large body of evidence highlights the beneficial effects of cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) on survival, but to date, there is little consensus on the optimal treatment strategy for patients with colorectal cancer peritoneal metastasis. The purpose of this study is to evaluate the impact of CRS + HIPEC on survival and provide reference for the treatment of patients with colorectal cancer peritoneal metastasis.
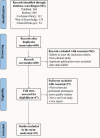
Methods: This systematic review and meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PubMed, Embase, Cochrane, Web of Knowledge, and ClinicalTrials.gov databases were screened from inception of the review to March 11, 2022. Ten studies were included in qualitative and quantitative analysis.
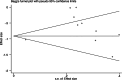
Results: A total of 3200 patients were enrolled in the study, including 788 patients in the CRS and HIPEC groups and 2412 patients in the control group, of which 3 were randomized controlled trials and 7 were cohort studies. The 3 randomized controlled studies were of high quality, and the quality scores of the 7 cohort studies were all 7 or above, indicating high quality. The results showed that the OS of CRS + HIPEC group was higher than that of control group (HR: 0.53, 95% CI: 0.38-0.73; P < 0.00001, I2 = 82.9%); the heterogeneity of the studies was large. The subgroup analysis showed that the OS of CRS and HIPEC group was higher than that of PC group (HR: 0.37, 95% CI: 0.30-0.47; P = 0.215, I2 = 31%) and higher than that in CRS group (HR: 0.73, 95% CI: 0.49-1.07; P = 0.163, I2 = 44.8%); the heterogeneity of the studies was low. In the OPEN group, the OS of THE CRS and HIPEC groups was higher than that in the control group (HR: 0.51, 95% CI: 0.38-0.70; P = 0.353, I2 = 3.9%); OPEN group showed lower heterogeneity. The OS of 60-100-min group was higher than that in the control group (HR: 0.65, 95% CI: 0.49-0.88; P = 0.172, I2 = 37.4%); the heterogeneity of the studies was low. Sensitivity analysis showed that there was no significant difference in the results of the combined analysis after each study was deleted. The results of publication bias showed that the P-value of Egger and Begg tests was 0.078 > 0.05, indicating that there is no publication bias.
Conclusions: CRS + HIPEC can improve the survival rate of patients with colorectal cancer peritoneal metastasis.
Keywords: Colorectal cancer; Cytoreductive surgery; Hyperthermic intraperitoneal chemotherapy; Meta-analysis; Peritoneal metastasis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
[Meta analysis of whether cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy can improve survival in patients with colorectal cancer peritoneal metastasis].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Mar 25;24(3):256-263. doi: 10.3760/cma.j.cn.441530-20201111-00604. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 34645170 Chinese.
-
[Efficacy of 1 384 cases of peritoneal carcinomatosis underwent cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy].Zhonghua Wei Chang Wai Ke Za Zhi. 2021 Mar 25;24(3):230-239. doi: 10.3760/cma.j.cn.441530-20201110-00603. Zhonghua Wei Chang Wai Ke Za Zhi. 2021. PMID: 34645167 Chinese.
-
Neoadjuvant chemotherapy followed by hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal metastasis: a retrospective study of its safety and efficacy.World J Surg Oncol. 2021 May 17;19(1):151. doi: 10.1186/s12957-021-02255-w. World J Surg Oncol. 2021. PMID: 34001125 Free PMC article.
-
Impact of Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in the Treatment of Gastric Cancer with Peritoneal Carcinomatosis: A Systematic Review and Meta-analysis.Ann Surg Oncol. 2022 Nov;29(12):7528-7537. doi: 10.1245/s10434-022-12312-7. Epub 2022 Aug 5. Ann Surg Oncol. 2022. PMID: 35930109
-
Indications for hyperthermic intraperitoneal chemotherapy with cytoreductive surgery: a systematic review.Eur J Cancer. 2020 Mar;127:76-95. doi: 10.1016/j.ejca.2019.10.034. Epub 2020 Jan 24. Eur J Cancer. 2020. PMID: 31986452
Cited by
-
Strategies for the comprehensive treatment of gastric cancer ovarian metastasis.World J Clin Oncol. 2025 Jun 24;16(6):106589. doi: 10.5306/wjco.v16.i6.106589. World J Clin Oncol. 2025. PMID: 40585825 Free PMC article. Review.
-
Repeat cytoreductive surgery with HIPEC for colorectal peritoneal metastases: a systematic review.World J Surg Oncol. 2024 Apr 17;22(1):99. doi: 10.1186/s12957-024-03386-6. World J Surg Oncol. 2024. PMID: 38627808 Free PMC article.
-
Diffuse malignant peritoneal mesothelioma: A review.Front Surg. 2023 Jan 6;9:1015884. doi: 10.3389/fsurg.2022.1015884. eCollection 2022. Front Surg. 2023. PMID: 36684194 Free PMC article. Review.
-
Survival Difference of Endometrial Cancer Patients with Peritoneal Metastasis Receiving Cytoreductive Surgery (CRS) with and without Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Systematic Review and Meta-Analysis.Int J Mol Sci. 2024 Jul 8;25(13):7495. doi: 10.3390/ijms25137495. Int J Mol Sci. 2024. PMID: 39000603 Free PMC article.
-
Expert consensus on the optimal management of BRAFV600E-mutant metastatic colorectal cancer in the Asia-Pacific region.Asia Pac J Clin Oncol. 2025 Feb;21(1):31-45. doi: 10.1111/ajco.14132. Epub 2024 Oct 25. Asia Pac J Clin Oncol. 2025. PMID: 39456063 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

