Engineering plants with carbon nanotubes: a sustainable agriculture approach
- PMID: 35701848
- PMCID: PMC9195285
- DOI: 10.1186/s12951-022-01483-w
Engineering plants with carbon nanotubes: a sustainable agriculture approach
Abstract
Sustainable agriculture is an important conception to meet the growing food demand of the global population. The increased need for adequate and safe food, as well as the ongoing ecological destruction associated with conventional agriculture practices are key global challenges. Nanomaterials are being developed in the agriculture sector to improve the growth and protection of crops. Among the various engineered nanomaterials, carbon nanotubes (CNTs) are one of the most promising carbon-based nanomaterials owing to their attractive physiochemical properties such as small size, high surface area, and superior mechanical and thermal strength, offering better opportunities for agriculture sector applications. This review provides basic information about CNTs, including their history; classification; and electrical, thermal, and mechanical properties, with a focus on their applications in the agriculture field. Furthermore, the mechanisms of the uptake and translocation of CNTs in plants and their defense mechanisms against environmental stresses are discussed. Finally, the major shortcomings, threats, and challenges of CNTs are assessed to provide a broad and clear view of the potential and future directions for CNT-based agriculture applications to achieve the goal of sustainability.
Keywords: Agriculture; Antimicrobial activity; Biosensors; Carbon nanotubes; Environmental stress; Gene delivery; Plant growth; Sustainability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
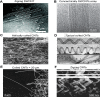


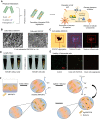




References
-
- Abhilash P, Tripathi V, Edrisi SA, Dubey RK, Bakshi M, Dubey PK, Singh H, Ebbs SD. Sustainability of crop production from polluted lands. Energy Ecol Environ. 2016;1:54–65. doi: 10.1007/s40974-016-0007-x. - DOI
-
- Acharya A, Pal PK. Agriculture nanotechnology: translating research outcome to field applications by influencing environmental sustainability. NanoImpact. 2020;19:100232. doi: 10.1016/j.impact.2020.100232. - DOI
-
- Pandey G. Challenges and future prospects of agri-nanotechnology for sustainable agriculture in India. Environ Technol Innov. 2018;11:299–307. doi: 10.1016/j.eti.2018.06.012. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

