Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer
- PMID: 35701865
- PMCID: PMC9357620
- DOI: 10.1111/cas.15462
Phase IIb study of pembrolizumab combined with S-1 + oxaliplatin or S-1 + cisplatin as first-line chemotherapy for gastric cancer
Abstract
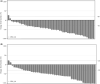
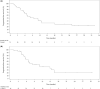
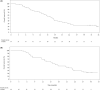
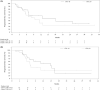
The KEYNOTE-659 study evaluated the efficacy and safety of first-line pembrolizumab plus S-1 and oxaliplatin (SOX) (cohort 1) or S-1 and cisplatin (SP) (cohort 2) for advanced gastric/gastroesophageal junction (G/GEJ) cancer in Japan. Herein, we update the results of cohort 1 and describe the results of cohort 2. This open-label phase IIb study enrolled patients with advanced programmed death-ligand 1 (PD-L1)-positive (combined positive score ≥ 1) human epidermal growth factor receptor 2 (HER2)-negative G/GEJ adenocarcinoma. The primary end-point was the objective response rate (ORR). Other end-points were duration of response (DOR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety. One hundred patients were enrolled. In cohorts 1 and 2, median follow-up time was 16.9 and 17.1 months; ORR (central review), 72.2% and 80.4%; DOR, 10.6 and 9.5 months; DCR (central review), 96.3% and 97.8%; median PFS (central review), 9.4 and 8.3 months; and median OS, 16.9 and 17.1 months, respectively. Treatment-related adverse events (TRAEs) occurred in all patients, including peripheral sensory neuropathy (94.4%, cohort 1), decreased neutrophil count (82.6%, cohort 2), nausea (59.3% and 60.9% in cohorts 1 and 2), and decreased appetite (61.1% and 60.9% in cohorts 1 and 2). Grade 3 or higher TRAEs were reported by 59.3% (cohort 1) and 78.3% (cohort 2), including decreased platelet count (14.8%, cohort 1) and decreased neutrophil count (52.2%, cohort 2). Pembrolizumab in combination with SOX or SP showed favorable efficacy and safety in patients with PD-L1-positive, HER2-negative G/GEJ adenocarcinoma.
Keywords: S-1; cisplatin; gastric cancer; oxaliplatin; pembrolizumab.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209‐249. - PubMed
-
- Cancer Information Service, National Cancer Center, Japan . Cancer registry and statistics. 2018. (in Japanese). https://ganjoho.jp/reg_stat/statistics/stat/summary.html. Accessed August 30, 2021.
-
- National Comprehensive Cancer Network . NCCN clinical practice guidelines in Oncology (NCCN Guidelines). Gastric Cancer Version 4. 2020. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf. Accessed September 15, 2021.
-
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;275:v38‐v49. - PubMed
-
- Muro K, Van Cutsem E, Narita Y, et al. Pan‐Asian adapted ESMO clinical practice guidelines for the management of patients with metastatic gastric cancer: a JSMO–ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30:19‐33. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

