Medications and Adherence to Treatment Guidelines Among Children Hospitalized With Acute COVID-19
- PMID: 35701866
- PMCID: PMC9444870
- DOI: 10.1542/peds.2022-056606
Medications and Adherence to Treatment Guidelines Among Children Hospitalized With Acute COVID-19
Abstract
Objectives: Coronavirus disease 2019 (COVID-19) treatment guidelines rapidly evolved during the pandemic. The December 2020 Infectious Diseases Society of America (IDSA) guideline, endorsed by the Pediatric Infectious Diseases Society, recommended steroids for critical disease, and suggested steroids and remdesivir for severe disease. We evaluated how medications for children hospitalized with COVID-19 changed after guideline publication.
Methods: We performed a multicenter, retrospective cohort study of children aged 30 days to <18 years hospitalized with acute COVID-19 at 42 tertiary care US children's hospitals April 2020 to December 2021. We compared medication use before and after the December 2020 IDSA guideline (pre- and postguideline) stratified by COVID-19 disease severity (mild-moderate, severe, critical) with interrupted time series.
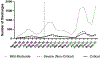
Results: Among 18 364 patients who met selection criteria, 80.3% were discharged in the postguideline period. Remdesivir and steroid use increased postguideline relative to the preguideline period, although the trend slowed. Postguideline, among patients with severe disease, 75.4% received steroids and 55.2% remdesivir, and in those with critical disease, 82.4% received steroids and 41.4% remdesivir. Compared with preguideline, enoxaparin use increased overall but decreased among patients with critical disease. Postguideline, tocilizumab use increased and hydroxychloroquine, azithromycin, anakinra, and antibiotic use decreased. Antibiotic use remained high in severe (51.7%) and critical disease (81%).
Conclusions: Although utilization of COVID-19 medications changed after December 2020 IDSA guidelines, there was a decline in uptake and incomplete adherence for children with severe and critical disease. Efforts should enhance reliable delivery of guideline-directed therapies to children hospitalized with COVID-19 and assess their effectiveness.
Copyright © 2022 by the American Academy of Pediatrics.
Figures


References
-
- American Academy of Pediatrics and Children’s Hospital Association. Children and COVID-19: State-level data report. American Academy of Pediatrics; 2022. Version April 7, 2022. Accessed April 11, 2022. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/...
-
- Centers for Disease Control and Prevention. COVID Data Tracker: Demographic Trends of COVID-19 cases and deaths in the US reported to CDC. Centers for Diease Control and Prevention; 2022. Updated April 11, 2022. Accessed April 11, 2022. https://covid.cdc.gov/covid-data-tracker/#demographics
-
- Bhimraj A, Morgan RL, Shumaker AH, et al. Overview of IDSA COVID-19 Treatment Guidelines (Summary Table). Version 5.6.0. Infectious Diseases Society of America; Nov 18, 2021. Accessed Jan 15, 2022. https://www.idsociety.org/COVID19guidelines#toc-1
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

