Piezo1-Regulated Mechanotransduction Controls Flow-Activated Lymphatic Expansion
- PMID: 35701867
- PMCID: PMC9308715
- DOI: 10.1161/CIRCRESAHA.121.320565
Piezo1-Regulated Mechanotransduction Controls Flow-Activated Lymphatic Expansion
Abstract
Background: Mutations in PIEZO1 (Piezo type mechanosensitive ion channel component 1) cause human lymphatic malformations. We have previously uncovered an ORAI1 (ORAI calcium release-activated calcium modulator 1)-mediated mechanotransduction pathway that triggers lymphatic sprouting through Notch downregulation in response to fluid flow. However, the identity of its upstream mechanosensor remains unknown. This study aimed to identify and characterize the molecular sensor that translates the flow-mediated external signal to the Orai1-regulated lymphatic expansion.
Methods: Various mutant mouse models, cellular, biochemical, and molecular biology tools, and a mouse tail lymphedema model were employed to elucidate the role of Piezo1 in flow-induced lymphatic growth and regeneration.
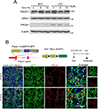
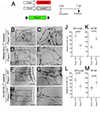
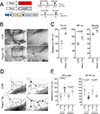
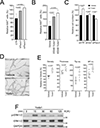
Results: Piezo1 was found to be abundantly expressed in lymphatic endothelial cells. Piezo1 knockdown in cultured lymphatic endothelial cells inhibited the laminar flow-induced calcium influx and abrogated the flow-mediated regulation of the Orai1 downstream genes, such as KLF2 (Krüppel-like factor 2), DTX1 (Deltex E3 ubiquitin ligase 1), DTX3L (Deltex E3 ubiquitin ligase 3L,) and NOTCH1 (Notch receptor 1), which are involved in lymphatic sprouting. Conversely, stimulation of Piezo1 activated the Orai1-regulated mechanotransduction in the absence of fluid flow. Piezo1-mediated mechanotransduction was significantly blocked by Orai1 inhibition, establishing the epistatic relationship between Piezo1 and Orai1. Lymphatic-specific conditional Piezo1 knockout largely phenocopied sprouting defects shown in Orai1- or Klf2- knockout lymphatics during embryo development. Postnatal deletion of Piezo1 induced lymphatic regression in adults. Ectopic Dtx3L expression rescued the lymphatic defects caused by Piezo1 knockout, affirming that the Piezo1 promotes lymphatic sprouting through Notch downregulation. Consistently, transgenic Piezo1 expression or pharmacological Piezo1 activation enhanced lymphatic sprouting. Finally, we assessed a potential therapeutic value of Piezo1 activation in lymphatic regeneration and found that a Piezo1 agonist, Yoda1, effectively suppressed postsurgical lymphedema development.
Conclusions: Piezo1 is an upstream mechanosensor for the lymphatic mechanotransduction pathway and regulates lymphatic growth in response to external physical stimuli. Piezo1 activation presents a novel therapeutic opportunity for preventing postsurgical lymphedema. The Piezo1-regulated lymphangiogenesis mechanism offers a molecular basis for Piezo1-associated lymphatic malformation in humans.
Keywords: calmodulin; endothelial cell; lymphedema; mechanotransduction, cellular; mutation.
Conflict of interest statement
Disclosures
Alex S. Huang discloses his relationships with Santen Pharmaceutical (Consultant), Allergan (Consultant), Aerie Pharmaceuticals (Consultant), Heidelberg Engineering (Research Support), Glaukos Corporation (Research Support), and Diagnosys (Research Support). All other authors disclose no conflict of interest with this study.
Figures







Comment in
-
Cation Channelopathies: Novel Insights into Generalized Lymphatic Dysplasia.Circ Res. 2022 Jul 8;131(2):130-132. doi: 10.1161/CIRCRESAHA.122.321400. Epub 2022 Jul 7. Circ Res. 2022. PMID: 35861738 Free PMC article. No abstract available.
References
-
- Escobedo N and Oliver G. Lymphangiogenesis: Origin, Specification, and Cell Fate Determination. Annu Rev Cell Dev Biol. 2016;32:677–691. - PubMed
-
- Choi D, Park E, Jung E, Seong YJ, Hong M, Lee S, Burford J, Gyarmati G, Peti-Peterdi J, Srikanth S, Gwack Y, Koh CJ, Boriushkin E, Hamik A, Wong AK and Hong YK. ORAI1 Activates Proliferation of Lymphatic Endothelial Cells in Response to Laminar Flow Through Kruppel-Like Factors 2 and 4. Circ Res. 2017;120:1426–1439. - PMC - PubMed
-
- Choi D, Park E, Jung E, Seong YJ, Yoo J, Lee E, Hong M, Lee S, Ishida H, Burford J, Peti-Peterdi J, Adams RH, Srikanth S, Gwack Y, Chen CS, Vogel HJ, Koh CJ, Wong AK and Hong YK. Laminar flow downregulates Notch activity to promote lymphatic sprouting. J Clin Invest. 2017;127:1225–1240. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

