Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure
- PMID: 35701874
- PMCID: PMC9685328
- DOI: 10.1161/CIRCRESAHA.121.319817
Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure
Erratum in
-
Correction to: Mitochondrial H2S Regulates BCAA Catabolism in Heart Failure.Circ Res. 2022 Sep 30;131(8):e101. doi: 10.1161/RES.0000000000000575. Epub 2022 Sep 29. Circ Res. 2022. PMID: 36173826 No abstract available.
Abstract
Background: Hydrogen sulfide (H2S) exerts mitochondria-specific actions that include the preservation of oxidative phosphorylation, biogenesis, and ATP synthesis, while inhibiting cell death. 3-MST (3-mercaptopyruvate sulfurtransferase) is a mitochondrial H2S-producing enzyme whose functions in the cardiovascular disease are not fully understood. In the current study, we investigated the effects of global 3-MST deficiency in the setting of pressure overload-induced heart failure.
Methods: Human myocardial samples obtained from patients with heart failure undergoing cardiac surgeries were probed for 3-MST protein expression. 3-MST knockout mice and C57BL/6J wild-type mice were subjected to transverse aortic constriction to induce pressure overload heart failure with reduced ejection fraction. Cardiac structure and function, vascular reactivity, exercise performance, mitochondrial respiration, and ATP synthesis efficiency were assessed. In addition, untargeted metabolomics were utilized to identify key pathways altered by 3-MST deficiency.
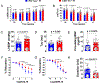
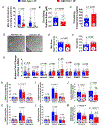
Results: Myocardial 3-MST was significantly reduced in patients with heart failure compared with nonfailing controls. 3-MST KO mice exhibited increased accumulation of branched-chain amino acids in the myocardium, which was associated with reduced mitochondrial respiration and ATP synthesis, exacerbated cardiac and vascular dysfunction, and worsened exercise performance following transverse aortic constriction. Restoring myocardial branched-chain amino acid catabolism with 3,6-dichlorobenzo1[b]thiophene-2-carboxylic acid (BT2) and administration of a potent H2S donor JK-1 ameliorates the detrimental effects of 3-MST deficiency in heart failure with reduced ejection fraction.
Conclusions: Our data suggest that 3-MST derived mitochondrial H2S may play a regulatory role in branched-chain amino acid catabolism and mediate critical cardiovascular protection in heart failure.
Keywords: amino acids, branched-chain; cell death; heart failure; hydrogen sulfide; mitochondrial respiration.
Conflict of interest statement
CONFLICT OF INTEREST DISCLOSURES
Dr. David Lefer serves as scientific consultant for Sulfagenix Inc, a company focusing on developing H2S-based therapy for clinical use. Dr. Lefer also has stock in both NovoMedix and SAJE Pharma, biotech companies that are developing novel therapeutics for cardiovascular diseases. Other authors declare no conflict of interests.
Figures





Comment in
-
Unbreak My Heart: Restore H2S and Branched Chain Amino Acid Oxidation in the Mitochondria.Circ Res. 2022 Jul 22;131(3):236-238. doi: 10.1161/CIRCRESAHA.122.321483. Epub 2022 Jul 21. Circ Res. 2022. PMID: 35862502 No abstract available.
References
-
- Bozkurt B, Hershberger RE, Butler J, Grady KL, Heidenreich PA, Isler ML, Kirklin JK and Weintraub WS. 2021 ACC/AHA Key Data Elements and Definitions for Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Heart Failure). Circ Cardiovasc Qual Outcomes. 2021;14:e000102. - PMC - PubMed
-
- Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG, American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–19. - PMC - PubMed
-
- Sun H and Wang Y. Branched chain amino acid metabolic reprogramming in heart failure. Biochim Biophys Acta. 2016;1862:2270–2275. - PubMed
-
- Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, Rhee J, Hoshino A, Kim B, Ibrahim A, Baca LG, Kim E, Ghosh CC, Parikh SM, Jiang A, Chu Q, Forman DE, Lecker SH, Krishnaiah S, Rabinowitz JD, Weljie AM, Baur JA, Kasper DL and Arany Z. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat Med. 2016;22:421–6. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

