A multiplex protein panel assay for severity prediction and outcome prognosis in patients with COVID-19: An observational multi-cohort study
- PMID: 35702332
- PMCID: PMC9181834
- DOI: 10.1016/j.eclinm.2022.101495
A multiplex protein panel assay for severity prediction and outcome prognosis in patients with COVID-19: An observational multi-cohort study
Abstract
Background: Global healthcare systems continue to be challenged by the COVID-19 pandemic, and there is a need for clinical assays that can help optimise resource allocation, support treatment decisions, and accelerate the development and evaluation of new therapies.
Methods: We developed a multiplexed proteomics assay for determining disease severity and prognosis in COVID-19. The assay quantifies up to 50 peptides, derived from 30 known and newly introduced COVID-19-related protein markers, in a single measurement using routine-lab compatible analytical flow rate liquid chromatography and multiple reaction monitoring (LC-MRM). We conducted two observational studies in patients with COVID-19 hospitalised at Charité - Universitätsmedizin Berlin, Germany before (from March 1 to 26, 2020, n=30) and after (from April 4 to November 19, 2020, n=164) dexamethasone became standard of care. The study is registered in the German and the WHO International Clinical Trials Registry (DRKS00021688).
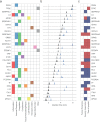
Findings: The assay produces reproducible (median inter-batch CV of 10.9%) absolute quantification of 47 peptides with high sensitivity (median LLOQ of 143 ng/ml) and accuracy (median 96.8%). In both studies, the assay reproducibly captured hallmarks of COVID-19 infection and severity, as it distinguished healthy individuals, mild, moderate, and severe COVID-19. In the post-dexamethasone cohort, the assay predicted survival with an accuracy of 0.83 (108/130), and death with an accuracy of 0.76 (26/34) in the median 2.5 weeks before the outcome, thereby outperforming compound clinical risk assessments such as SOFA, APACHE II, and ABCS scores.
Interpretation: Disease severity and clinical outcomes of patients with COVID-19 can be stratified and predicted by the routine-applicable panel assay that combines known and novel COVID-19 biomarkers. The prognostic value of this assay should be prospectively assessed in larger patient cohorts for future support of clinical decisions, including evaluation of sample flow in routine setting. The possibility to objectively classify COVID-19 severity can be helpful for monitoring of novel therapies, especially in early clinical trials.
Funding: This research was funded in part by the European Research Council (ERC) under grant agreement ERC-SyG-2020 951475 (to M.R) and by the Wellcome Trust (IA 200829/Z/16/Z to M.R.). The work was further supported by the Ministry of Education and Research (BMBF) as part of the National Research Node 'Mass Spectrometry in Systems Medicine (MSCoresys)', under grant agreements 031L0220 and 161L0221. J.H. was supported by a Swiss National Science Foundation (SNSF) Postdoc Mobility fellowship (project number 191052). This study was further supported by the BMBF grant NaFoUniMedCOVID-19 - NUM-NAPKON, FKZ: 01KX2021. The study was co-funded by the UK's innovation agency, Innovate UK, under project numbers 75594 and 56328.
Keywords: Biomarker; COVID-19; Clinical disease progression; Disease prognosis; LC-MS/MS; Machine learning; SARS-CoV2; Severity stratification; Targeted proteomics.
© 2022 The Authors.
Conflict of interest statement
EM Scientific Limited (t/a Inoviv) and Charité – Universitätsmedizin Berlin (Ziyue Wang, Michael Mülleder, Vadim Demichev, Johannes Hartl and Markus Ralser) filed joint patent applications for the protein panel assay described herein - United States Application No: 63/156291, 63/283787 and 17/685756. Leif-Erik Sander received honoraria for lectures from Boehringer Ingelheim, Novartis, Berlin Chemie, GSK, Merck, Novartis, Sanofi. Ernestas Sirka and Adam Cryar are/were employees of EM Scientific Limited (t/a Inoviv). Daniel Blake, Rebekah L Sayers and Catherine S Lane are employees of SCIEX. Christoph Mueller and Johannes Zeiser are employees of Agilent Technologies.
Figures




References
-
- Kearns P, Siebert S, Willicombe M, et al. Examining the Immunological Effects of COVID-19 Vaccination in Patients with Conditions Potentially Leading to Diminished Immune Response Capacity – The OCTAVE Trial 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

