The chemistry of snake venom and its medicinal potential
- PMID: 35702592
- PMCID: PMC9185726
- DOI: 10.1038/s41570-022-00393-7
The chemistry of snake venom and its medicinal potential
Abstract
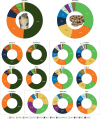
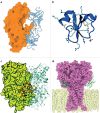
The fascination and fear of snakes dates back to time immemorial, with the first scientific treatise on snakebite envenoming, the Brooklyn Medical Papyrus, dating from ancient Egypt. Owing to their lethality, snakes have often been associated with images of perfidy, treachery and death. However, snakes did not always have such negative connotations. The curative capacity of venom has been known since antiquity, also making the snake a symbol of pharmacy and medicine. Today, there is renewed interest in pursuing snake-venom-based therapies. This Review focuses on the chemistry of snake venom and the potential for venom to be exploited for medicinal purposes in the development of drugs. The mixture of toxins that constitute snake venom is examined, focusing on the molecular structure, chemical reactivity and target recognition of the most bioactive toxins, from which bioactive drugs might be developed. The design and working mechanisms of snake-venom-derived drugs are illustrated, and the strategies by which toxins are transformed into therapeutics are analysed. Finally, the challenges in realizing the immense curative potential of snake venom are discussed, and chemical strategies by which a plethora of new drugs could be derived from snake venom are proposed.
Keywords: Biochemistry; Drug discovery.
© Springer Nature Limited 2022.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

