ANGT_HUMAN[448-462], an Anorexigenic Peptide Identified Using Plasma Peptidomics
- PMID: 35702602
- PMCID: PMC9184509
- DOI: 10.1210/jendso/bvac082
ANGT_HUMAN[448-462], an Anorexigenic Peptide Identified Using Plasma Peptidomics
Abstract
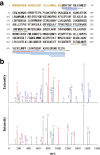
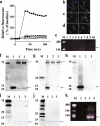
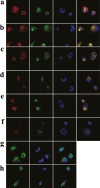
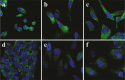
The discovery of bioactive peptides is an important research target that enables the elucidation of the pathophysiology of human diseases and provides seeds for drug discovery. Using a large number of native peptides previously identified using plasma peptidomics technology, we sequentially synthesized selected sequences and subjected them to functional screening using human cultured cells. A 15-amino-acid residue proangiotensinogen-derived peptide, designated ANGT_HUMAN[448-462], elicited cellular responses and bound to cultured human cells. Synthetic fluorescent-labeled and biotinylated ANGT_HUMAN[448-462] peptides were rendered to bind to cell- and tissue-derived proteins and peptide-cell protein complexes were retrieved and analyzed using liquid chromatography-tandem mass spectrometry, revealing the β-subunit of ATP synthase as its cell-surface binding protein. Because ATP synthase mediates the effects of anorexigenic peptides, the ability of ANGT_HUMAN[448-462] to modulate eating behavior in mice was investigated. Both intraperitoneal and intracerebroventricular injections of low doses of ANGT_HUMAN[448-462] suppressed spontaneous food and water intake throughout the dark phase of the diurnal cycle without affecting locomotor activity. Immunoreactive ANGT_HUMAN[448-462], distributed throughout human tissues and in human-derived cells, is mostly co-localized with angiotensin II and is occasionally present separately from angiotensin II. In this study, an anorexigenic peptide, ANGT_HUMAN[448-462], was identified by exploring cell surface target proteins of the human native peptides identified using plasma peptidomics.
Keywords: ANGT_HUMAN[448–462]; angiotensinogen; anorectic peptide; food intake; liquid chromatography–tandem mass spectrometry; plasma peptidomics.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures



References
-
- Saito T, Kawashima Y, Minamida S, et al. Establishment and application of a high- quality comparative analysis strategy for the discovery and small-scale validation of low-abundance biomarker peptides in serum based on an optimized novel peptide extraction method. J Electrophoresis. 2013;57(1):1-9. doi:10.2198/jelectroph.57.1
LinkOut - more resources
Full Text Sources
Research Materials
