Brain alterations in the early Alzheimer's continuum with amyloid-β, tau, glial and neurodegeneration CSF markers
- PMID: 35702732
- PMCID: PMC9185381
- DOI: 10.1093/braincomms/fcac134
Brain alterations in the early Alzheimer's continuum with amyloid-β, tau, glial and neurodegeneration CSF markers
Abstract
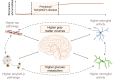
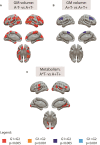
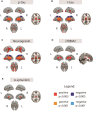
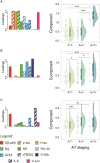
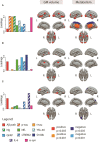
Higher grey matter volumes/cortical thickness and fluorodeoxyglucose uptake have been consistently found in cognitively unimpaired individuals with abnormal Alzheimer's disease biomarkers compared with those with normal biomarkers. It has been hypothesized that such transient increases may be associated with neuroinflammatory mechanisms triggered in response to early Alzheimer's pathology. Here, we evaluated, in the earliest stages of the Alzheimer's continuum, associations between grey matter volume and fluorodeoxyglucose uptake with CSF biomarkers of several pathophysiological mechanisms known to be altered in preclinical Alzheimer's disease stages. We included 319 cognitively unimpaired participants from the ALFA+ cohort with available structural MRI, fluorodeoxyglucose PET and CSF biomarkers of amyloid-β and tau pathology (phosphorylated tau and total tau), synaptic dysfunction (neurogranin), neuronal and axonal injury (neurofilament light), glial activation (soluble triggering receptor on myeloid cells 2, YKL40, GFAP, interleukin-6 and S100b) and α-synuclein using the Roche NeuroToolKit. We first used the amyloid-β/tau framework to investigate differences in the neuroimaging biomarkers between preclinical Alzheimer's disease stages. Then, we looked for associations between the neuroimaging markers and all the CSF markers. Given the non-negative nature of the concentrations of CSF biomarkers and their high collinearity, we clustered them using non-negative matrix factorization approach (components) and sought associations with the imaging markers. By groups, higher grey matter volumes were found in the amyloid-β-positive tau-negative participants with respect to the reference amyloid-β-negative tau-negative group. Both amyloid-β and tau-positive participants showed higher fluorodeoxyglucose uptake than tau-negative individuals. Using the obtained components, we observed that tau pathology accompanied by YKL-40 (astrocytic marker) was associated with higher grey matter volumes and fluorodeoxyglucose uptake in extensive brain areas. Higher grey matter volumes in key Alzheimer-related regions were also found in association with two other components characterized by a higher expression of amyloid-β in combination with different glial markers: one with higher GFAP and S100b levels (astrocytic markers) and the other one with interleukin-6 (pro-inflammatory). Notably, these components' expression had different behaviours across amyloid-β/tau stages. Taken together, our results show that CSF amyloid-β and phosphorylated tau, in combination with different aspects of glial response, have distinctive associations with higher grey matter volumes and increased glucose metabolism in key Alzheimer-related regions. These mechanisms combine to produce transient higher grey matter volumes and fluorodeoxyglucose uptake at the earliest stages of the Alzheimer's continuum, which may revert later on the course of the disease when neurodegeneration drives structural and metabolic cerebral changes.
Keywords: cerebrospinal fluid biomarkers; fluorodeoxyglucose positron emission tomography; neurodegeneration; neuroinflammation; structural magnetic resonance imaging.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Chételat G, Villemagne VL, Pike KE, et al. . Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–3358. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
