FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity
- PMID: 35703180
- PMCID: PMC9197528
- DOI: 10.1172/JCI157574
FOXA2 suppresses endometrial carcinogenesis and epithelial-mesenchymal transition by regulating enhancer activity
Abstract
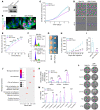
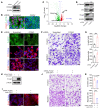
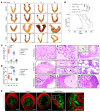
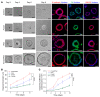
FOXA2 encodes a transcription factor mutated in 10% of endometrial cancers (ECs), with a higher mutation rate in aggressive variants. FOXA2 has essential roles in embryonic and uterine development. However, FOXA2's role in EC is incompletely understood. Functional investigations using human and mouse EC cell lines revealed that FOXA2 controls endometrial epithelial gene expression programs regulating cell proliferation, adhesion, and endometrial-epithelial transition. In live animals, conditional inactivation of Foxa2 or Pten alone in endometrial epithelium did not result in ECs, but simultaneous inactivation of both genes resulted in lethal ECs with complete penetrance, establishing potent synergism between Foxa2 and PI3K signaling. Studies in tumor-derived cell lines and organoids highlighted additional invasion and cell growth phenotypes associated with malignant transformation and identified key mediators, including Myc and Cdh1. Transcriptome and cistrome analyses revealed that FOXA2 broadly controls gene expression programs through modification of enhancer activity in addition to regulating specific target genes, rationalizing its tumor suppressor functions. By integrating results from our cell lines, organoids, animal models, and patient data, our findings demonstrated that FOXA2 is an endometrial tumor suppressor associated with aggressive disease and with shared commonalities among its roles in endometrial function and carcinogenesis.
Keywords: Cancer; Molecular pathology; Oncology; Tumor suppressors.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

