Preclinical Profile of Gadoquatrane: A Novel Tetrameric, Macrocyclic High Relaxivity Gadolinium-Based Contrast Agent
- PMID: 35703267
- PMCID: PMC9444293
- DOI: 10.1097/RLI.0000000000000889
Preclinical Profile of Gadoquatrane: A Novel Tetrameric, Macrocyclic High Relaxivity Gadolinium-Based Contrast Agent
Abstract
Objectives: The aim of this report was to characterize the key physicochemical, pharmacokinetic (PK), and magnetic resonance imaging (MRI) properties of gadoquatrane (BAY 1747846), a newly designed tetrameric, macrocyclic, extracellular gadolinium-based contrast agent (GBCA) with high relaxivity and stability.
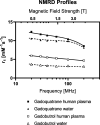
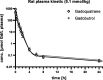
Materials and methods: The r1-relaxivities of the tetrameric gadoquatrane at 1.41 and 3.0 T were determined in human plasma and the nuclear magnetic relaxation dispersion profiles in water and plasma. The complex stability was analyzed in human serum over 21 days at pH 7.4 at 37°C and was compared with the linear GBCA gadodiamide and the macrocyclic GBCA (mGBCA) gadobutrol. In addition, zinc transmetallation assay was performed to investigate the kinetic inertness. Protein binding and the blood-to-plasma ratio were determined in vitro using rat and human plasma. The PK profile was evaluated in rats (up to 7 days postinjection). Magnetic resonance imaging properties were investigated using a glioblastoma (GS9L) rat model.
Results: The new chemical entity gadoquatrane is a macrocyclic tetrameric Gd complex with one inner sphere water molecule per Gd ( q = 1). Gadoquatrane showed high solubility in buffer (1.43 mol Gd/L, 10 mM Tris-HCl, pH 7.4), high hydrophilicity (logP -4.32 in 1-butanol/water), and negligible protein binding. The r1-relaxivity of gadoquatrane in human plasma per Gd of 11.8 mM -1 ·s -1 (corresponding to 47.2 mM -1 ·s -1 per molecule at 1.41 T at 37°C, pH 7.4) was more than 2-fold (8-fold per molecule) higher compared with established mGBCAs. Nuclear magnetic relaxation dispersion profiles confirmed the more than 2-fold higher r1-relaxivity in human plasma for the clinically relevant magnetic field strengths from 0.47 to 3.0 T. The complex stability of gadoquatrane at physiological conditions was very high. The observed Gd release after 21 days at 37°C in human serum was below the lower limit of quantification. Gadoquatrane showed no Gd 3+ release in the presence of zinc in the transmetallation assay. The PK profile (plasma elimination, biodistribution, recovery) was comparable to that of gadobutrol. In MRI, the quantitative evaluation of the tumor-to-brain contrast in the rat glioblastoma model showed significantly improved contrast enhancement using gadoquatrane compared with gadobutrol at the same Gd dose administered (0.1 mmol Gd/kg body weight). In comparison to gadoterate meglumine, similar contrast enhancement was reached with gadoquatrane with 75% less Gd dose. In terms of the molecule dose, this was reduced by 90% when compared with gadoterate meglumine. Because of its tetrameric structure and hence lower number of molecules per volume, all prepared formulations of gadoquatrane were iso-osmolar to blood.
Conclusions: The tetrameric gadoquatrane is a novel, highly effective mGBCA for use in MRI. Gadoquatrane provides favorable physicochemical properties (high relaxivity and stability, negligible protein binding) while showing essentially the same PK profile (fast extracellular distribution, fast elimination via the kidneys in an unchanged form) to established mGBCAs on the market. Overall, gadoquatrane is an excellent candidate for further clinical development.
Trial registration: ClinicalTrials.gov NCT05061979.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Conflicts of interest and sources of funding: J.L., M.B., T.F., C.-S. H., G.J., O.P., M.B., W.E., and H.P. are employees of Bayer AG. O.P. and M.B. were employees of Bayer AG at the time of this investigation.
Figures




References
-
- Caravan P Ellison JJ McMurry TJ, et al. . Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. - PubMed
-
- Sieber MA Lengsfeld P Frenzel T, et al. . Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18:2164–2173. - PubMed
-
- Frenzel T Lengsfeld P Schirmer H, et al. . Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–828. - PubMed
-
- Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

