Topical application of Porphyromonas gingivalis into the gingival pocket in mice leads to chronic‑active infection, periodontitis and systemic inflammation
- PMID: 35703359
- PMCID: PMC9242655
- DOI: 10.3892/ijmm.2022.5159
Topical application of Porphyromonas gingivalis into the gingival pocket in mice leads to chronic‑active infection, periodontitis and systemic inflammation
Abstract
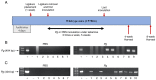
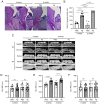
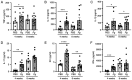
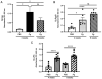
Porphyromonas gingivalis (Pg), one of the 'red‑complex' perio‑pathogens known to play a critical role in the development of periodontitis, has been used in various animal models to mimic human bacteria‑induced periodontitis. In order to achieve a more realistic animal model of human Pg infection, the present study investigated whether repeated small‑volume topical applications of Pg directly into the gingival pocket can induce local infection, including periodontitis and systemic vascular inflammation in wild‑type mice. Freshly cultured Pg was topically applied directly into the gingival pocket of the second molars for 5 weeks (3 times/week). After the final application, the mice were left in cages for 4 or 8 weeks and sacrificed. The status of Pg colony formation in the pocket, gingival inflammation, alveolar bone loss, the expression levels of pro‑inflammatory cytokines in the serum and aorta, the presence of anti‑Pg lipopolysaccharide (LPS) and gingipain (Kpg and RgpB) antibodies in the serum, as well as the accumulation of Pg LPS and gingipain aggregates in the gingiva and arterial wall were evaluated. The topical application of Pg into the gingival pocket induced the following local and systemic pathohistological changes in mice when examined at 4 or 8 weeks after the final topical Pg application: Pg colonization in the majority of gingival pockets; increased gingival pocket depths; gingival inflammation indicated by the increased expression of TNF‑α, IL‑6 and IL‑1β; significant loss of alveolar bone at the sites of topical Pg application; and increased levels of pro‑inflammatory cytokines, such as TNF‑α, IL‑1β, IL‑17, IL‑13, KC and IFN‑γ in the serum in comparison to those from mice receiving PBS. In addition, the Pg application/colonization model induced anti‑Pg LPS and gingipain antibodies in serum, as well as the accumulation of Pg LPS and gingipain aggregates in the gingivae and arterial walls. To the best of our knowledge, this mouse model represents the first example of creating a more sustained local infection in the gingival tissues of wild‑type mice and may prove to be useful for the investigation of the more natural and complete pathogenesis of the bacteria in the development of local oral and systemic diseases, such as atherosclerosis. It may also be useful for the determination of a treatment/prevention/efficacy model associated with Pg‑induced colonization periodontitis in mice.
Keywords: Porphyromonas gingivalis; gingipains; intrapocket infection; periodontitis; systemic inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Proteolytic Activity-Independent Activation of the Immune Response by Gingipains from Porphyromonas gingivalis.mBio. 2022 Jun 28;13(3):e0378721. doi: 10.1128/mbio.03787-21. Epub 2022 May 2. mBio. 2022. PMID: 35491845 Free PMC article.
-
The role of gingipains in the pathogenesis of periodontal disease.J Periodontol. 2003 Jan;74(1):111-8. doi: 10.1902/jop.2003.74.1.111. J Periodontol. 2003. PMID: 12593605 Review.
-
rgpA-Engineered/Functionalized DNA Vaccine as a Novel Prophylactic Vaccination to Prevent Porphyromonas gingivalis-Induced Periodontitis: An in Vivo Study.Discov Med. 2024 Feb;36(181):355-365. doi: 10.24976/Discov.Med.202436181.33. Discov Med. 2024. PMID: 38409840
-
Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-α and interleukin-1β but suppresses that by interleukin-17A: importance of proteolytic degradation of osteoprotegerin by lysine gingipain.J Biol Chem. 2014 May 30;289(22):15621-30. doi: 10.1074/jbc.M113.520510. Epub 2014 Apr 22. J Biol Chem. 2014. PMID: 24755218 Free PMC article.
-
Porphyromonas gingivalis lipopolysaccharide signaling in gingival fibroblasts-CD14 and Toll-like receptors.Crit Rev Oral Biol Med. 2002;13(2):132-42. doi: 10.1177/154411130201300204. Crit Rev Oral Biol Med. 2002. PMID: 12097356 Review.
Cited by
-
GV1001, hTERT Peptide Fragment, Prevents Doxorubicin-Induced Endothelial-to-Mesenchymal Transition in Human Endothelial Cells and Atherosclerosis in Mice.Cells. 2025 Jan 10;14(2):98. doi: 10.3390/cells14020098. Cells. 2025. PMID: 39851526 Free PMC article.
-
Periodontitis-induced neuroinflammation triggers IFITM3-Aβ axis to cause alzheimer's disease-like pathology and cognitive decline.Alzheimers Res Ther. 2025 Jul 19;17(1):166. doi: 10.1186/s13195-025-01818-3. Alzheimers Res Ther. 2025. PMID: 40684176 Free PMC article.
-
Impact of allogeneic dental pulp stem cell injection on tissue regeneration in periodontitis: a multicenter randomized clinical trial.Signal Transduct Target Ther. 2025 Jul 31;10(1):239. doi: 10.1038/s41392-025-02320-w. Signal Transduct Target Ther. 2025. PMID: 40739139 Free PMC article. Clinical Trial.
-
hTERT Peptide Fragment GV1001 Prevents the Development of Porphyromonas gingivalis-Induced Periodontal Disease and Systemic Disorders in ApoE-Deficient Mice.Int J Mol Sci. 2024 Jun 1;25(11):6126. doi: 10.3390/ijms25116126. Int J Mol Sci. 2024. PMID: 38892314 Free PMC article.
-
The interplay between oral microbiota, gut microbiota and systematic diseases.J Oral Microbiol. 2023 May 15;15(1):2213112. doi: 10.1080/20002297.2023.2213112. eCollection 2023. J Oral Microbiol. 2023. PMID: 37200866 Free PMC article. Review.
References
-
- Takii R, Kadowaki T, Baba A, Tsukuba T, Yamamoto K. A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems. Infect Immun. 2005;73:883–893. doi: 10.1128/IAI.73.2.883-893.2005. - DOI - PMC - PubMed

