Anti-Spike Antibody Response to Natural Infection with SARS-CoV-2 and Its Activity against Emerging Variants
- PMID: 35703556
- PMCID: PMC9430469
- DOI: 10.1128/spectrum.00743-22
Anti-Spike Antibody Response to Natural Infection with SARS-CoV-2 and Its Activity against Emerging Variants
Abstract
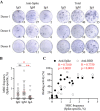
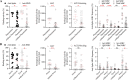
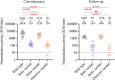
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has substantially affected human health globally. Spike-specific antibody response plays a major role in protection against SARS-CoV-2 infection. Here, we examined serological anti-spike antibody and memory B cell responses in adults with acute SARS-CoV-2 infection. Twenty-five adult patients were enrolled between January and September 2020, and 21 (84%) had a detectable spike-binding antibody response in serum on day 21 ± 8 (6 to 33) after the onset of illness. Among those with positive spike-binding antibody response, 19 (90%) had a positive hemagglutination titer and 15 (71%) had angiotensin-converting enzyme 2 (ACE2)-blocking serological activities. Follow-up serum samples collected 11 ± 1 (7 to 15) months after infection exhibited an average of 2.6 ± 1.0 (1.0 to 3.5)-fold reduction in the spike-binding antibody response. Moreover, convalescent and follow-up serum samples showed 83 ± 82 (15 to 306)- and 165 ± 167 (12 to 456)-fold reductions in the neutralization activity against the Omicron variant, respectively. Upon acute infection, spike-specific memory B cell responses were elicited, with an average frequency of 1.3% ± 1.2% of peripheral B cells on day 19 ± 7 (6 to 33) after the onset of illness. IgM memory B cells were predominantly induced. Patients with fever and pneumonia showed significantly stronger spike-binding, ACE2-blocking antibody, and memory B cell responses. In conclusion, spike-specific antibody response elicited upon acute SARS-CoV-2 infection may wane over time and be compromised by the emergence of viral variants. IMPORTANCE As spike protein-specific antibody responses play a major role in protection against SARS-CoV-2, we examined spike-binding and ACE2-blocking antibody responses in SARS-CoV-2 infection at different time points. We found robust responses following acute infection, which waned approximately 11 months after infection. Patients with fever and pneumonia showed significantly stronger spike-binding, ACE2-blocking antibody, and memory B cell responses. In particular, spike-specific antibody response in the convalescent and follow-up serum samples was substantially affected by emerging variants, especially Beta and Omicron variants. These results warrant continued surveillance of spike-specific antibody responses to natural infections and highlight the importance of maintaining functional anti-spike antibodies through immunization.
Keywords: COVID-19; Omicron; SARS-CoV-2; memory B cell; neutralization antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Competitive SARS-CoV-2 Serology Reveals Most Antibodies Targeting the Spike Receptor-Binding Domain Compete for ACE2 Binding.mSphere. 2020 Sep 16;5(5):e00802-20. doi: 10.1128/mSphere.00802-20. mSphere. 2020. PMID: 32938700 Free PMC article.
-
Sequential Analysis of Binding and Neutralizing Antibody in COVID-19 Convalescent Patients at 14 Months After SARS-CoV-2 Infection.Front Immunol. 2021 Nov 26;12:793953. doi: 10.3389/fimmu.2021.793953. eCollection 2021. Front Immunol. 2021. PMID: 34899762 Free PMC article.
-
A Glycosylated RBD Protein Induces Enhanced Neutralizing Antibodies against Omicron and Other Variants with Improved Protection against SARS-CoV-2 Infection.J Virol. 2022 Sep 14;96(17):e0011822. doi: 10.1128/jvi.00118-22. Epub 2022 Aug 16. J Virol. 2022. PMID: 35972290 Free PMC article.
-
[SARS-CoV-2 neutralizing monoclonal antibodies and nanobodies: a review].Sheng Wu Gong Cheng Xue Bao. 2022 Sep 25;38(9):3173-3193. doi: 10.13345/j.cjb.220328. Sheng Wu Gong Cheng Xue Bao. 2022. PMID: 36151792 Review. Chinese.
-
Angiotensin-Converting Enzyme 2 (ACE2) in the Pathogenesis of ARDS in COVID-19.Front Immunol. 2021 Dec 22;12:732690. doi: 10.3389/fimmu.2021.732690. eCollection 2021. Front Immunol. 2021. PMID: 35003058 Free PMC article. Review.
Cited by
-
Presence of neutralizing SARS-CoV-2 antibodies in asymptomatic population of N'Djamena, Chad.Immun Inflamm Dis. 2024 Jan;12(1):e1154. doi: 10.1002/iid3.1154. Immun Inflamm Dis. 2024. PMID: 38270301 Free PMC article.
-
Safety, Tolerability, and Immunogenicity of Booster Dose with MVC-COV1901 or MVC-COV1901-Beta SARS-CoV-2 Vaccine in Adults: A Phase I, Prospective, Randomized, Open-Labeled Study.Vaccines (Basel). 2023 Dec 1;11(12):1798. doi: 10.3390/vaccines11121798. Vaccines (Basel). 2023. PMID: 38140202 Free PMC article.
-
Dynamics of humoral immune response in SARS-CoV-2 infected individuals with different clinical stages.Front Immunol. 2022 Nov 14;13:1007068. doi: 10.3389/fimmu.2022.1007068. eCollection 2022. Front Immunol. 2022. PMID: 36451829 Free PMC article.
-
Humoral immunity and safety of respiratory virus vaccines in systemic lupus erythematosus population: a meta-analysis based on twenty-five observational studies.Ann Med. 2024 Dec;56(1):2392882. doi: 10.1080/07853890.2024.2392882. Epub 2024 Aug 19. Ann Med. 2024. PMID: 39155852 Free PMC article.
-
COVID-19 Seroprevalence in Romania: Insights from a Nationwide Antibody Study.Epidemiologia (Basel). 2025 Jun 4;6(2):26. doi: 10.3390/epidemiologia6020026. Epidemiologia (Basel). 2025. PMID: 40558600 Free PMC article.
References
-
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. 2020. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. doi:10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
-
- World Health Organization. 2020. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). World Health Organization, Geneva, Switzerland. https://www.who.int/groups/covid-19-ihr-emergency-committee. Accessed 6 February 2022.
-
- Done E, Du I, Garder L. 2022. COVID-19 dashboard by the Centre for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). John Hopkins University, Baltimore, MD. https://coronavirus.jhu.edu. Accessed 6 February 2022.
-
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. 2020. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280. doi:10.1016/j.cell.2020.02.052. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

