Dehydroepiandrosterone alleviates hypoxia-induced learning and memory dysfunction by maintaining synaptic homeostasis
- PMID: 35703574
- PMCID: PMC9344085
- DOI: 10.1111/cns.13869
Dehydroepiandrosterone alleviates hypoxia-induced learning and memory dysfunction by maintaining synaptic homeostasis
Abstract
Aims: Hypoxia causes plenty of pathologies in the central nervous system (CNS) including impairment of cognitive and memory function. Dehydroepiandrosterone (DHEA) has been proved to have therapeutic effects on CNS injuries by maintaining the homeostasis of synapses, yet its effect on hypoxia-induced CNS damage remains unknown.
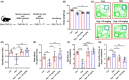
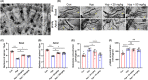
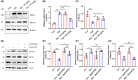
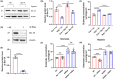
Methods: In vivo and in vitro models were established. Concentrations of glutamate and γ GABA were tested by ELISA. Levels of synapse-associated proteins were measured by western blotting. Density of dendritic protrusions of hippocampal neurons was assessed by Golgi staining. Immunofluorescence was adopted to observe the morphology of primary neurons. The novel object recognition test (NORT) and shuttle box test were used to evaluate cognition.
Results: Dehydroepiandrosterone reversed abnormal elevation of glutamate levels, shortenings of neuronal processes, decreases in the density of dendritic protrusions, downregulation of synaptosome-associated protein (SNAP25), and impaired cognition caused by hypoxia. Hypoxia also resulted in notably downregulation of syntaxin 1A (Stx-1A). Overexpression of Stx-1A dramatically attenuated hypoxia-induced elevation of glutamate. Treatment with DHEA reversed the Stx-1A downregulation caused by hypoxic exposure.
Conclusion: Dehydroepiandrosterone may exert a protective effect on hypoxia-induced memory impairment by maintaining synaptic homeostasis. These findings offer a novel understanding of the therapeutic effect of DHEA on hypoxia-induced cognitive dysfunction.
Keywords: dehydroepiandrosterone; hippocampus; hypoxia; neuron; synaptic function.
© 2022 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Biselli R, Farrace S, D'Amelio R, Fattorossi A. Influence of stress on lymphocyte subset distribution—a flow cytometric study in young student pilots. Aviat Space Environ Med. 1993;64(2):116‐120. - PubMed
-
- Burek M, König A, Lang M, et al. Hypoxia‐induced MicroRNA‐212/132 Alter blood‐brain barrier integrity through inhibition of tight junction‐associated proteins in human and mouse brain microvascular endothelial cells. Transl Stroke Res. 2019;10(6):672‐683. doi: 10.1007/s12975-018-0683-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

