Glucosamine amends CNS pathology in mucopolysaccharidosis IIIC mouse expressing misfolded HGSNAT
- PMID: 35704026
- PMCID: PMC9204472
- DOI: 10.1084/jem.20211860
Glucosamine amends CNS pathology in mucopolysaccharidosis IIIC mouse expressing misfolded HGSNAT
Abstract
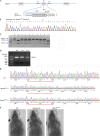
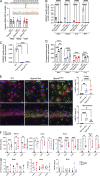
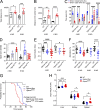
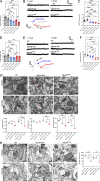
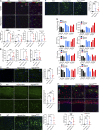
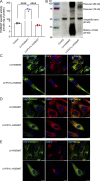
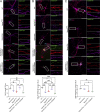
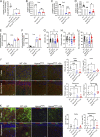
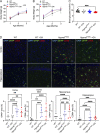
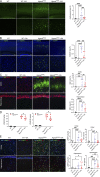
The majority of mucopolysaccharidosis IIIC (MPS IIIC) patients have missense variants causing misfolding of heparan sulfate acetyl-CoA:α-glucosaminide N-acetyltransferase (HGSNAT), which are potentially treatable with pharmacological chaperones. To test this approach, we generated a novel HgsnatP304L mouse model expressing misfolded HGSNAT Pro304Leu variant. HgsnatP304L mice present deficits in short-term and working/spatial memory 2-4 mo earlier than previously described constitutive knockout Hgsnat-Geo mice. HgsnatP304L mice also show augmented severity of neuroimmune response, synaptic deficits, and neuronal storage of misfolded proteins and gangliosides compared with Hgsnat-Geo mice. Expression of misfolded human Pro311Leu HGSNAT protein in cultured hippocampal Hgsnat-Geo neurons further reduced levels of synaptic proteins. Memory deficits and majority of brain pathology were rescued in mice receiving HGSNAT chaperone, glucosamine. Our data for the first time demonstrate dominant-negative effects of misfolded HGSNAT Pro304Leu variant and show that they are treatable by oral administration of glucosamine. This suggests that patients affected with mutations preventing normal folding of the enzyme can benefit from chaperone therapy.
© 2022 Pan et al.
Conflict of interest statement
Disclosures: A.V. Pshezhetsky reported personal fees from Phoenix Nest Inc. and grants from Phoenix Nest Inc. outside the submitted work. No other disclosures were reported.
Figures


References
-
- Akkerman, S., Blokland A., Reneerkens O., van Goethem N.P., Bollen E., Gijselaers H.J., Lieben C.K., Steinbusch H.W., and Prickaerts J.. 2012. Object recognition testing: Methodological considerations on exploration and discrimination measures. Behav. Brain Res. 232:335–347. 10.1016/j.bbr.2012.03.022 - DOI - PubMed
-
- Amegandjin, C.A., Choudhury M., Jadhav V., Carrico J.N., Quintal A., Berryer M., Snapyan M., Chattopadhyaya B., Saghatelyan A., and Di Cristo G.. 2021. Sensitive period for rescuing parvalbumin interneurons connectivity and social behavior deficits caused by TSC1 loss. Nat. Commun. 12:3653. 10.1038/s41467-021-23939-7 - DOI - PMC - PubMed
-
- Asano, N., Ishii S., Kizu H., Ikeda K., Yasuda K., Kato A., Martin O.R., and Fan J.Q.. 2000. In vitro inhibition and intracellular enhancement of lysosomal alpha-galactosidase A activity in Fabry lymphoblasts by 1-deoxygalactonojirimycin and its derivatives. Eur. J. Biochem. 267:4179–4186. 10.1046/j.1432-1327.2000.01457.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

