Impact of Pruritus on Quality of Life and Current Treatment Patterns in Patients with Primary Biliary Cholangitis
- PMID: 35704252
- PMCID: PMC10406656
- DOI: 10.1007/s10620-022-07581-x
Impact of Pruritus on Quality of Life and Current Treatment Patterns in Patients with Primary Biliary Cholangitis
Erratum in
-
Correction to: Impact of Pruritus on Quality of Life and Current Treatment Patterns in Patients with Primary Biliary Cholangitis.Dig Dis Sci. 2023 Oct;68(10):4064-4065. doi: 10.1007/s10620-023-08050-9. Dig Dis Sci. 2023. PMID: 37555884 Free PMC article. No abstract available.
Abstract
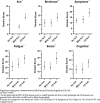
Background and aims: Patients with primary biliary cholangitis (PBC) often suffer with pruritus. We describe the impact of pruritus on quality of life and how it is managed in a real-world cohort.
Methods: TARGET-PBC is a longitudinal observational cohort of patients with PBC across the USA. Data include information from medical records for three years prior to the date of consent up to 5 years of follow-up. Enrolled patients were asked to complete patient-reported outcome surveys: PBC-40, 5-D itch, and the PROMIS fatigue survey. Kruskal-Wallis tests were used to compare differences in symptoms between groups.
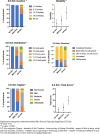
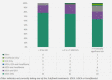
Results: A total of 211 patients with completed PRO surveys were included in the current study. PRO respondents were compared with non-respondents in the TARGET-PBC population and were broadly similar. Pruritus was reported in 170 patients (81%), with those reporting clinically significant pruritus (30%) scoring worse across each domain of the PBC-40 and 5-D itch, more frequently having cirrhosis, and having significantly greater levels of fatigue. Patients reporting clinically significant pruritus were more likely to receive treatment, but 33% had never received treatment (no itch = 43.9%, mild itch = 38.3%).
Conclusions: The prevalence of pruritus was high in this population, and those reporting clinically significant pruritus had a higher likelihood of having advanced disease and worse quality of life. However, this study found that pruritus in PBC is under-treated. This may be due in part to ineffectiveness of current treatments, poor tolerance, or the lack of FDA-approved medications for pruritus.
Keywords: Primary Biliary Cholangitis; Pruritus; Real-world evidence; Treatment.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
MJM has research funding from TARGET PharmaSolutions, GlaxoSmithKline, Intercept Pharmaceuticals, CymaBay Pharmaceuticals, Genfit, Mirum, and Mallinckrodt; and consultation fees from TARGET PharmaSolutions, GlaxoSmithKline, CymaBay Pharmaceuticals, and Mallinckrodt. EC and WRK have no conflicts to report. HS and MM are employees of GlaxoSmithKline and hold stocks/shares in the company. AT was an employee of GSK at the time of the study and holds stocks/shares in the company. ARM, HLM, and RS are Target RWE employees. CB has research funding from Gilead Biosciences, Intercept Pharmaceuticals, CymaBay Pharmaceuticals, Takeda Pharmaceuticals, GlaxoSmithKline, BristolMyerSquibb, TARGET Pharmasolutions, Novartis, BiomX, Mirum, Genfit, Pliant, Cara Therapeutics, and Boston Scientific. CL has research funding from Gilead, Intercept, CymaBay, Genfit, GSK, Novartis, High Tide, Zydus, Cara Therapeutics, Mirum, Pliant, and Target PharmaSolutions; consultation fees from CymaBay, Genfit, GSK, Pliant, Mirum, Cara Therapeutics, Escient, Teva, Calliditas, and Intercept; royalties from Up-to-date; and is the Associate Editor for Hepatology, Member of the ABIM Test and Policy committee for transplant hepatology.
Figures
References
-
- Hirschfield G, et al. ENHance: safety and efficacy of seladelpar in patients with primary biliary cholangitis (pbc)-a phase 3 international, randomized, placebo-controlled study. in The Liver Meeting Digital Experience™. 2020. AASLD.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials