Rivaroxaban Monotherapy vs Combination Therapy With Antiplatelets on Total Thrombotic and Bleeding Events in Atrial Fibrillation With Stable Coronary Artery Disease: A Post Hoc Secondary Analysis of the AFIRE Trial
- PMID: 35704345
- PMCID: PMC9201742
- DOI: 10.1001/jamacardio.2022.1561
Rivaroxaban Monotherapy vs Combination Therapy With Antiplatelets on Total Thrombotic and Bleeding Events in Atrial Fibrillation With Stable Coronary Artery Disease: A Post Hoc Secondary Analysis of the AFIRE Trial
Abstract
Importance: Appropriate regimens of antithrombotic therapy for patients with atrial fibrillation (AF) and coronary artery disease (CAD) have not yet been established.
Objective: To compare the total number of thrombotic and/or bleeding events between rivaroxaban monotherapy and combined rivaroxaban and antiplatelet therapy in such patients.
Design, setting, and participants: This was a post hoc secondary analysis of the Atrial Fibrillation and Ischemic Events With Rivaroxaban in Patients With Stable Coronary Artery Disease (AFIRE) open-label, randomized clinical trial. This multicenter analysis was conducted from February 23, 2015, to July 31, 2018. Patients with AF and stable CAD who had undergone percutaneous coronary intervention or coronary artery bypass grafting 1 or more years earlier or who had angiographically confirmed CAD not requiring revascularization were enrolled. Data were analyzed from September 1, 2020, to March 26, 2021.
Interventions: Rivaroxaban monotherapy or combined rivaroxaban and antiplatelet therapy.
Main outcomes and measures: The total incidence of thrombotic, bleeding, and fatal events was compared between the groups. Cox regression analyses were used to estimate the risk of subsequent events in the 2 groups, with the status of thrombotic or bleeding events that had occurred by the time of death used as a time-dependent variable.
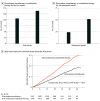
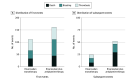
Results: A total of 2215 patients (mean [SD] age, 74 [8.2] years; 1751 men [79.1%]) were included in the modified intention-to-treat analysis. The total event rates for the rivaroxaban monotherapy group (1107 [50.0%]) and the combination-therapy group (1108 [50.0%]) were 12.2% (135 of 1107) and 19.2% (213 of 1108), respectively, during a median follow-up of 24.1 (IQR, 17.3-31.5) months. The mortality rate was 3.7% (41 of 1107) in the monotherapy group and 6.6% (73 of 1108) in the combination-therapy group. Rivaroxaban monotherapy was associated with a lower risk of total events compared with combination therapy (hazard ratio, 0.62; 95% CI, 0.48-0.80; P < .001). Monotherapy was an independent factor associated with a lower risk of subsequent events compared with combination therapy. The mortality risk after a bleeding event (monotherapy, 75% [6 of 8]; combination therapy, 62.1% [18 of 29]) was higher than that after a thrombotic event (monotherapy, 25% [2 of 8]; combination therapy, 37.9% [11 of 29]).
Conclusions and relevance: Rivaroxaban monotherapy was associated with lower risks of total thrombotic and/or bleeding events than combination therapy in patients with AF and stable CAD. Tapered antithrombotic therapy with a sole anticoagulant should be considered in these patients.
Trial registration: ClinicalTrials.gov Identifier: NCT02642419.
Conflict of interest statement
Figures
Comment in
-
Antithrombotic Therapy in Atrial Fibrillation and Coronary Artery Disease: Does Less Mean More?JAMA Cardiol. 2022 Aug 1;7(8):794-795. doi: 10.1001/jamacardio.2022.1572. JAMA Cardiol. 2022. PMID: 35704350 No abstract available.
References
-
- Greenberg B, Butler J, Felker GM, et al. . Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387(10024):1178-1186. doi:10.1016/S0140-6736(16)00082-9 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

