Total Synthesis of Kibdelomycin
- PMID: 35704446
- PMCID: PMC9357209
- DOI: 10.1002/anie.202206183
Total Synthesis of Kibdelomycin
Abstract
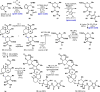
A modular total synthesis of kibdelomycin is disclosed that should enable structure-activity relationship (SAR) studies of this interesting class of antibiotics. The route uses simple building blocks and addresses lingering questions about its structural assignment and relationship to amycolamicin, a recently described natural product reported to have a similar structure. Initial antibacterial assays reveal that both C-22 epimers (the N-glycosidic linkage) of the natural product have similar activity while structurally truncated analogs lose activity.
Keywords: Antibiotics; Glycosidation; Modular Synthesis; Total Synthesis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Phillips J. W., et al., Chem. Biol. 2011, 18, 955–965. - PubMed
-
- None
-
- None
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

