CD38 reduces mitochondrial fitness and cytotoxic T cell response against viral infection in lupus patients by suppressing mitophagy
- PMID: 35704572
- PMCID: PMC9200274
- DOI: 10.1126/sciadv.abo4271
CD38 reduces mitochondrial fitness and cytotoxic T cell response against viral infection in lupus patients by suppressing mitophagy
Abstract
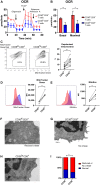
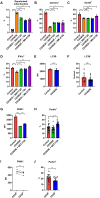
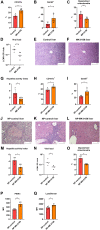
Infection is one of the major causes of mortality in patients with systemic lupus erythematosus (SLE). We previously found that CD38, an ectoenzyme that regulates the production of NAD+, is up-regulated in CD8+ T cells of SLE patients and correlates with the risk of infection. Here, we report that CD38 reduces CD8+ T cell function by negatively affecting mitochondrial fitness through the inhibition of multiple steps of mitophagy, a process that is critical for mitochondria quality control. Using a murine lupus model, we found that administration of a CD38 inhibitor in a CD8+ T cell-targeted manner reinvigorated their effector function, reversed the defects in autophagy and mitochondria, and improved viral clearance. We conclude that CD38 represents a target to mitigate infection rates in people with SLE.
Figures


References
-
- Tsokos G. C., Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011). - PubMed
-
- Kang I., Quan T., Nolasco H., Park S. H., Hong M. S., Crouch J., Pamer E. G., Howe J. G., Craft J., Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J. Immunol. 172, 1287–1294 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

