Leishmania braziliensis causing human disease in Northeast Brazil presents loci with genotypes in long-term equilibrium
- PMID: 35704664
- PMCID: PMC9239440
- DOI: 10.1371/journal.pntd.0010390
Leishmania braziliensis causing human disease in Northeast Brazil presents loci with genotypes in long-term equilibrium
Abstract
Background: Leishmaniases are neglected tropical diseases that inflict great burden to poor areas of the globe. Intense research has aimed to identify parasite genetic signatures predictive of infection outcomes. Consistency of diagnostic tools based on these markers would greatly benefit from accurate understanding of Leishmania spp. population genetics. We explored two chromosomal loci to characterize a population of L. braziliensis causing human disease in Northeast Brazil.
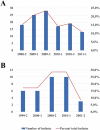
Methodology/principal findings: Two temporally distinct samples of L. braziliensis were obtained from patients attending the leishmaniasis clinic at the village of Corte de Pedra: (2008-2011) primary sample, N = 120; (1999-2001) validation sample, N = 35. Parasites were genotyped by Sanger's sequencing of two 600 base pairs loci starting at nucleotide positions 3,074 and 425,451 of chromosomes 24 and 28, respectively. Genotypes based on haplotypes of biallelic positions in each locus were tested for several population genetic parameters as well as for geographic clustering within the region. Ample geographic overlap of genotypes at the two loci was observed as indicated by non-significant Cusick and Edward's comparisons. No linkage disequilibrium was detected among combinations of haplotypes for both parasite samples. Homozygous and heterozygous genotypes displayed Hardy-Weinberg equilibrium (HWE) at both loci in the two samples when straight observed and expected counts were compared by Chi-square (p>0.5). However, Bayesian statistics using one million Monte-Carlo randomizations disclosed a less robust HWE for chromosome 24 genotypes, particularly in the primary sample (p = 0.04). Fixation indices (Fst) were consistently lower than 0.05 among individuals of the two samples at both tested loci, and no intra-populational structuralization could be detected using STRUCTURE software.
Conclusions/significance: These findings suggest that L. braziliensis can maintain stable populations in foci of human leishmaniasis and are capable of robust genetic recombination possibly due to events of sexual reproduction during the parasite's lifecycle.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Hotez PJ, Bottazzi ME, Franco-Paredes C, Ault SK, Periago MR. The neglected tropical diseases of Latin America and the Caribbean: a review of disease burden and distribution and a roadmap for control and elimination. PLoS Negl Trop Dis. 2008/09/30. 2008;2: e300. doi: 10.1371/journal.pntd.0000300 - DOI - PMC - PubMed
-
- Guimaraes LH, Machado PR, Lago EL, Morgan DJ, Schriefer A, Bacellar O, et al. Atypical manifestations of tegumentary leishmaniasis in a transmission area of Leishmania braziliensis in the state of Bahia, Brazil. Trans R Soc Trop Med Hyg. 2009/06/02. 2009;103: 712–715. doi: 10.1016/j.trstmh.2009.04.019 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

