Nuclear speckle integrity and function require TAO2 kinase
- PMID: 35704758
- PMCID: PMC9231605
- DOI: 10.1073/pnas.2206046119
Nuclear speckle integrity and function require TAO2 kinase
Abstract
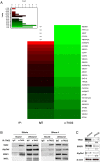
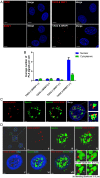
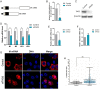
Nuclear speckles are non-membrane-bound organelles known as storage sites for messenger RNA (mRNA) processing and splicing factors. More recently, nuclear speckles have also been implicated in splicing and export of a subset of mRNAs, including the influenza virus M mRNA that encodes proteins required for viral entry, trafficking, and budding. However, little is known about how nuclear speckles are assembled or regulated. Here, we uncovered a role for the cellular protein kinase TAO2 as a constituent of nuclear speckles and as a factor required for the integrity of these nuclear bodies and for their functions in pre-mRNA splicing and trafficking. We found that a nuclear pool of TAO2 is localized at nuclear speckles and interacts with nuclear speckle factors involved in RNA splicing and nuclear export, including SRSF1 and Aly/Ref. Depletion of TAO2 or inhibition of its kinase activity disrupts nuclear speckle structure, decreasing the levels of several proteins involved in nuclear speckle assembly and splicing, including SC35 and SON. Consequently, splicing and nuclear export of influenza virus M mRNA were severely compromised and caused a disruption in the virus life cycle. In fact, low levels of TAO2 led to a decrease in viral protein levels and inhibited viral replication. Additionally, depletion or inhibition of TAO2 resulted in abnormal expression of a subset of mRNAs with key roles in viral replication and immunity. Together, these findings uncovered a function of TAO2 in nuclear speckle formation and function and revealed host requirements and vulnerabilities for influenza infection.
Keywords: TAOK2; mRNA export; nuclear speckles; splicing.
Conflict of interest statement
Competing interest statement: The A.G.-S. laboratory has received research support from Pfizer, Senhwa Biosciences, Kenall Manufacturing, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines, Atea Pharma, and Merck, outside of the reported work. A.G.-S. has consulting agreements with the following companies involving cash and/or stock: Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Vaxalto, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, Paratus, CureLab Oncology, CureLab Veterinary, Synairgen, and Pfizer, outside of the reported work. A.G.-S. has been an invited speaker at meeting events organized by Sequirus, Janssen, and AstraZeneca. A.G.-S. is an inventor on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, outside of the reported work.
Figures


References
-
- Ule J., Blencowe B. J., Alternative splicing regulatory networks: Functions, mechanisms, and evolution. Mol. Cell 76, 329–345 (2019). - PubMed
-
- Thompson M. G., Lynch K. W., Functional and mechanistic interplay of host and viral alternative splicing regulation during influenza infection. Cold Spring Harb. Symp. Quant. Biol. 84, 123–131 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

