Hebbian activity-dependent plasticity in white matter
- PMID: 35705046
- PMCID: PMC9376741
- DOI: 10.1016/j.celrep.2022.110951
Hebbian activity-dependent plasticity in white matter
Abstract
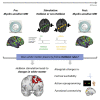
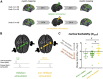
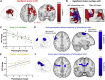
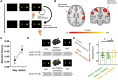
Synaptic plasticity is required for learning and follows Hebb's rule, the computational principle underpinning associative learning. In recent years, a complementary type of brain plasticity has been identified in myelinated axons, which make up the majority of brain's white matter. Like synaptic plasticity, myelin plasticity is required for learning, but it is unclear whether it is Hebbian or whether it follows different rules. Here, we provide evidence that white matter plasticity operates following Hebb's rule in humans. Across two experiments, we find that co-stimulating cortical areas to induce Hebbian plasticity leads to relative increases in cortical excitability and associated increases in a myelin marker within the stimulated fiber bundle. We conclude that Hebbian plasticity extends beyond synaptic changes and can be observed in human white matter fibers.
Keywords: CP: Neuroscience; Hebbian plasticity; action reprogramming; brain plasticity; brain stimulation; magnetic resonance imaging; myelin; myelin plasticity; white matter.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
Similar articles
-
Myelin plasticity modulates neural circuitry required for learning and behavior.Neurosci Res. 2021 Jun;167:11-16. doi: 10.1016/j.neures.2020.12.005. Epub 2021 Jan 5. Neurosci Res. 2021. PMID: 33417972 Review.
-
Donald O. Hebb's synapse and learning rule: a history and commentary.Neurosci Biobehav Rev. 2005 Jan;28(8):851-74. doi: 10.1016/j.neubiorev.2004.09.009. Neurosci Biobehav Rev. 2005. PMID: 15642626 Review.
-
Homeostatic role of heterosynaptic plasticity: models and experiments.Front Comput Neurosci. 2015 Jul 13;9:89. doi: 10.3389/fncom.2015.00089. eCollection 2015. Front Comput Neurosci. 2015. PMID: 26217218 Free PMC article. Review.
-
Muscarinic acetylcholine receptors control baseline activity and Hebbian stimulus timing-dependent plasticity in fusiform cells of the dorsal cochlear nucleus.J Neurophysiol. 2017 Mar 1;117(3):1229-1238. doi: 10.1152/jn.00270.2016. Epub 2016 Dec 21. J Neurophysiol. 2017. PMID: 28003407 Free PMC article.
-
White Matter Plasticity in the Adult Brain.Neuron. 2017 Dec 20;96(6):1239-1251. doi: 10.1016/j.neuron.2017.11.026. Neuron. 2017. PMID: 29268094 Free PMC article. Review.
Cited by
-
The mouse motor system contains multiple premotor areas and partially follows human organizational principles.Cell Rep. 2024 May 28;43(5):114191. doi: 10.1016/j.celrep.2024.114191. Epub 2024 May 7. Cell Rep. 2024. PMID: 38717901 Free PMC article.
-
A longitudinal resource for population neuroscience of school-age children and adolescents in China.Sci Data. 2023 Aug 21;10(1):545. doi: 10.1038/s41597-023-02377-8. Sci Data. 2023. PMID: 37604823 Free PMC article.
-
Cerebellar Activity Affects Distal Cortical Physiology and Synaptic Plasticity in a Human Parietal-Motor Pathway Associated with Motor Actions.J Neurosci. 2025 Jun 4;45(23):e0404252025. doi: 10.1523/JNEUROSCI.0404-25.2025. J Neurosci. 2025. PMID: 40306961
-
[Myelination as a modulating factor in memory circuitry].Rev Neurol. 2023 Feb 1;76(3):101-109. doi: 10.33588/rn.7603.2022325. Rev Neurol. 2023. PMID: 36703503 Free PMC article. Review. Spanish.
-
Repeated spaced paired-associative stimulation to the parietal-motor pathway maintains corticomotor excitability in older adults.Clin Neurophysiol. 2025 May;173:76-85. doi: 10.1016/j.clinph.2025.03.003. Epub 2025 Mar 8. Clin Neurophysiol. 2025. PMID: 40085997
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

