Absence of microglia promotes diverse pathologies and early lethality in Alzheimer's disease mice
- PMID: 35705056
- PMCID: PMC9285116
- DOI: 10.1016/j.celrep.2022.110961
Absence of microglia promotes diverse pathologies and early lethality in Alzheimer's disease mice
Abstract
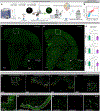
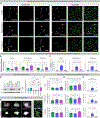
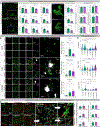
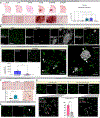
Microglia are strongly implicated in the development and progression of Alzheimer's disease (AD), yet their impact on pathology and lifespan remains unclear. Here we utilize a CSF1R hypomorphic mouse to generate a model of AD that genetically lacks microglia. The resulting microglial-deficient mice exhibit a profound shift from parenchymal amyloid plaques to cerebral amyloid angiopathy (CAA), which is accompanied by numerous transcriptional changes, greatly increased brain calcification and hemorrhages, and premature lethality. Remarkably, a single injection of wild-type microglia into adult mice repopulates the microglial niche and prevents each of these pathological changes. Taken together, these results indicate the protective functions of microglia in reducing CAA, blood-brain barrier dysfunction, and brain calcification. To further understand the clinical implications of these findings, human AD tissue and iPSC-microglia were examined, providing evidence that microglia phagocytose calcium crystals, and this process is impaired by loss of the AD risk gene, TREM2.
Keywords: Alzheimer’s disease; Alzheimer’s disease co-pathologies; CP: Neuroscience; TREM2; brain calcification; cerebral amyloid angiopathy; hemorrhage; iPSC-microglia; microglia; mortality; neurovascular.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.B.-J. is a co-inventor of patent application WO/2018/160496, related to the differentiation of pluripotent stem cells into microglia, and co-founder of NovoGlia. All other authors declare no competing interests.
Figures



References
-
- Arnold TD, Niaudet C, Pang M-F, Siegenthaler J, Gaengel K, Jung B, Ferrero GM, Mukouyama Y.s., Fuxe J, Akhurst R, et al. (2014). Excessive vascular sprouting underlies cerebral hemorrhage in mice lacking αVβ8-TGFβ signaling in the brain. Dev. Camb. Engl 141, 4489–4499. 10.1242/dev.107193. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

