Sodium-calcium exchanger-3 regulates pain "wind-up": From human psychophysics to spinal mechanisms
- PMID: 35705078
- PMCID: PMC7613464
- DOI: 10.1016/j.neuron.2022.05.017
Sodium-calcium exchanger-3 regulates pain "wind-up": From human psychophysics to spinal mechanisms
Abstract
Repeated application of noxious stimuli leads to a progressively increased pain perception; this temporal summation is enhanced in and predictive of clinical pain disorders. Its electrophysiological correlate is "wind-up," in which dorsal horn spinal neurons increase their response to repeated nociceptor stimulation. To understand the genetic basis of temporal summation, we undertook a GWAS of wind-up in healthy human volunteers and found significant association with SLC8A3 encoding sodium-calcium exchanger type 3 (NCX3). NCX3 was expressed in mouse dorsal horn neurons, and mice lacking NCX3 showed normal, acute pain but hypersensitivity to the second phase of the formalin test and chronic constriction injury. Dorsal horn neurons lacking NCX3 showed increased intracellular calcium following repetitive stimulation, slowed calcium clearance, and increased wind-up. Moreover, virally mediated enhanced spinal expression of NCX3 reduced central sensitization. Our study highlights Ca2+ efflux as a pathway underlying temporal summation and persistent pain, which may be amenable to therapeutic targeting.
Keywords: GWAS; central sensitization; in vivo calcium imaging; in vivo electrophysiology; pain; spinal cord; temporal summation; wind-up.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

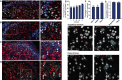



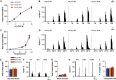
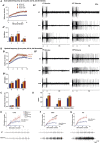

Comment in
-
Protecting against summation of pain.Neuron. 2022 Aug 17;110(16):2513-2515. doi: 10.1016/j.neuron.2022.07.008. Neuron. 2022. PMID: 35981521
References
-
- Adhikari K., Fuentes-Guajardo M., Quinto-Sánchez M., Mendoza-Revilla J., Camilo Chacón-Duque J., Acuña-Alonzo V., Jaramillo C., Arias W., Lozano R.B., Pérez G.M., et al. A genome-wide association scan implicates DCHS2, RUNX2, GLI3, PAX1 and EDAR in human facial variation. Nat. Commun. 2016;7:11616. doi: 10.1038/ncomms11616. - DOI - PMC - PubMed
-
- Adhikari K., Mendoza-Revilla J., Sohail A., Fuentes-Guajardo M., Lampert J., Chacón-Duque J.C., Hurtado M., Villegas V., Granja V., Acuña-Alonzo V., et al. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation in Eurasia. Nat. Commun. 2019;10:358. doi: 10.1038/s41467-018-08147-0. - DOI - PMC - PubMed
-
- Alsulaiman W.A.A., Quillet R., Bell A.M., Dickie A.C., Polgár E., Boyle K.A., Watanabe M., Roome R.B., Kania A., Todd A.J., et al. Characterisation of lamina I anterolateral system neurons that express Cre in a Phox2a-Cre mouse line. Sci. Rep. 2021;11:17912. doi: 10.1038/s41598-021-97105-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

