Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC
- PMID: 35705368
- PMCID: PMC9751228
- DOI: 10.1136/gutjnl-2021-326500
Protective and aggressive bacterial subsets and metabolites modify hepatobiliary inflammation and fibrosis in a murine model of PSC
Abstract
Objective: Conflicting microbiota data exist for primary sclerosing cholangitis (PSC) and experimental models.
Goal: define the function of complex resident microbes and their association relevant to PSC patients by studying germ-free (GF) and antibiotic-treated specific pathogen-free (SPF) multidrug-resistant 2 deficient (mdr2-/- ) mice and microbial profiles in PSC patient cohorts.
Design: We measured weights, liver enzymes, RNA expression, histological, immunohistochemical and fibrotic biochemical parameters, faecal 16S rRNA gene profiling and metabolomic endpoints in gnotobiotic and antibiotic-treated SPF mdr2-/- mice and targeted metagenomic analysis in PSC patients.
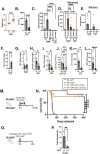
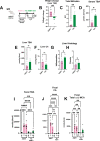
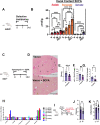
Results: GF mdr2-/- mice had 100% mortality by 8 weeks with increasing hepatic bile acid (BA) accumulation and cholestasis. Early SPF autologous stool transplantation rescued liver-related mortality. Inhibition of ileal BA transport attenuated antibiotic-accelerated liver disease and decreased total serum and hepatic BAs. Depletion of vancomycin-sensitive microbiota exaggerated hepatobiliary disease. Vancomycin selectively decreased Lachnospiraceae and short-chain fatty acids (SCFAs) but expanded Enterococcus and Enterobacteriaceae. Antibiotics increased Enterococcus faecalis and Escherichia coli liver translocation. Colonisation of GF mdr2-/- mice with translocated E. faecalis and E. coli strains accelerated hepatobiliary inflammation and mortality. Lachnospiraceae colonisation of antibiotic pretreated mdr2-/- mice reduced liver fibrosis, inflammation and translocation of pathobionts, and SCFA-producing Lachnospiraceae and purified SCFA decreased fibrosis. Faecal Lachnospiraceae negatively associated, and E. faecalis/ Enterobacteriaceae positively associated, with PSC patients' clinical severity by Mayo risk scores.
Conclusions: We identified novel functionally protective and detrimental resident bacterial species in mdr2-/- mice and PSC patients with associated clinical risk score. These insights may guide personalised targeted therapeutic interventions in PSC patients.
Keywords: antibiotics; cholestatic liver diseases; intestinal microbiology; primary sclerosing cholangitis; short chain fatty acids.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RBS has consulted for and received grant support from Takeda, Janssen, Second Genome, Vedanta, BiomX, Biomica, SERES and Artizan; JRH has served on advisory boards and/or given lectures for Orkla Health, Novartis, Amgen and Roche, and received research support from Biogen, all unrelated to the present study.
Figures






Comment in
-
Bacteria as key players in primary sclerosing cholangitis?Gut. 2023 Apr;72(4):607-608. doi: 10.1136/gutjnl-2022-327876. Epub 2022 Jun 28. Gut. 2023. PMID: 35764378 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK108959/DK/NIDDK NIH HHS/United States
- P30 DK034987/DK/NIDDK NIH HHS/United States
- I01 CX001076/CX/CSRD VA/United States
- K01 DK119582/DK/NIDDK NIH HHS/United States
- I01 BX005730/BX/BLRD VA/United States
- IS1 BX004777/BX/BLRD VA/United States
- U19 AI067798/AI/NIAID NIH HHS/United States
- I01 BX003031/BX/BLRD VA/United States
- T32 DK007737/DK/NIDDK NIH HHS/United States
- R01 DK115377/DK/NIDDK NIH HHS/United States
- I01 BX004033/BX/BLRD VA/United States
- R35 CA232109/CA/NCI NIH HHS/United States
- P30 ES010126/ES/NIEHS NIH HHS/United States
- R21 TR003095/TR/NCATS NIH HHS/United States
- I01 BX001328/BX/BLRD VA/United States
- P01 DK094779/DK/NIDDK NIH HHS/United States
- R21 AA026629/AA/NIAAA NIH HHS/United States
- IK6 BX004477/BX/BLRD VA/United States
- P40 OD010995/OD/NIH HHS/United States
- R01 DK119421/DK/NIDDK NIH HHS/United States
- IK6 BX005226/BX/BLRD VA/United States
- R01 DK104893/DK/NIDDK NIH HHS/United States
- R01 DK057543/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
