Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases
- PMID: 35705369
- PMCID: PMC9872249
- DOI: 10.1136/gutjnl-2021-325808
Blockade of interleukin 10 potentiates antitumour immune function in human colorectal cancer liver metastases
Abstract
Objective: Programmed cell death protein 1 (PD-1) checkpoint inhibition and adoptive cellular therapy have had limited success in patients with microsatellite stable colorectal cancer liver metastases (CRLM). We sought to evaluate the effect of interleukin 10 (IL-10) blockade on endogenous T cell and chimeric antigen receptor T (CAR-T) cell antitumour function in CRLM slice cultures.
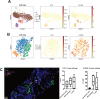
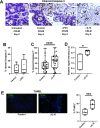
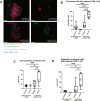
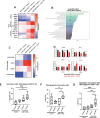
Design: We created organotypic slice cultures from human CRLM (n=38 patients' tumours) and tested the antitumour effects of a neutralising antibody against IL-10 (αIL-10) both alone as treatment and in combination with exogenously administered carcinoembryonic antigen (CEA)-specific CAR-T cells. We evaluated slice cultures with single and multiplex immunohistochemistry, in situ hybridisation, single-cell RNA sequencing, reverse-phase protein arrays and time-lapse fluorescent microscopy.
Results: αIL-10 generated a 1.8-fold increase in T cell-mediated carcinoma cell death in human CRLM slice cultures. αIL-10 significantly increased proportions of CD8+ T cells without exhaustion transcription changes, and increased human leukocyte antigen - DR isotype (HLA-DR) expression of macrophages. The antitumour effects of αIL-10 were reversed by major histocompatibility complex class I or II (MHC-I or MHC-II) blockade, confirming the essential role of antigen presenting cells. Interrupting IL-10 signalling also rescued murine CAR-T cell proliferation and cytotoxicity from myeloid cell-mediated immunosuppression. In human CRLM slices, αIL-10 increased CEA-specific CAR-T cell activation and CAR-T cell-mediated cytotoxicity, with nearly 70% carcinoma cell apoptosis across multiple human tumours. Pretreatment with an IL-10 receptor blocking antibody also potentiated CAR-T function.
Conclusion: Neutralising the effects of IL-10 in human CRLM has therapeutic potential as a stand-alone treatment and to augment the function of adoptively transferred CAR-T cells.
Keywords: COLORECTAL METASTASES; IMMUNOLOGY; IMMUNOTHERAPY; INTERLEUKINS; LIVER METASTASES.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: VGP is a member of the scientific advisory board for TriSalus Life Sciences. He has served as a consultant for Merck & Company in 2018, GlaxoSmithKline in 2019, Imvax in 2019, Takeda in 2020 and Umoja in 2022. He has research funding from AstraZeneca, Ipsen, Merck, OncoResponse and NGM. The funders had no role in the conceptualisation, design, data collection, analysis, decision to publish or preparation of the manuscript. The anti-CEA CAR vector was provided by TNK Therapeutics, who had no role in the conceptualisation, design, data collection, analysis or preparation of the manuscript. SCK served as an advisor for TNK Therapeutics until December 2019, is currently Chief Medical Officer of TriSalus Life Sciences and SAB member of Nkarta, Takeda and Sentibio, and has received research funding from Takeda and Nkata.
Figures


References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
