Graded Variation in T1w/T2w Ratio during Adolescence: Measurement, Caveats, and Implications for Development of Cortical Myelin
- PMID: 35705486
- PMCID: PMC9302463
- DOI: 10.1523/JNEUROSCI.2380-21.2022
Graded Variation in T1w/T2w Ratio during Adolescence: Measurement, Caveats, and Implications for Development of Cortical Myelin
Abstract
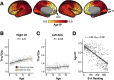
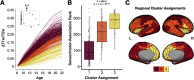
Adolescence is characterized by the maturation of cortical microstructure and connectivity supporting complex cognition and behavior. Axonal myelination influences brain connectivity during development by enhancing neural signaling speed and inhibiting plasticity. However, the maturational timing of cortical myelination during human adolescence remains poorly understood. Here, we take advantage of recent advances in high-resolution cortical T1w/T2w mapping methods, including principled correction of B1+ transmit field effects, using data from the Human Connectome Project in Development (HCP-D; N = 628, ages 8-21). We characterize microstructural changes relevant to myelination by estimating age-related differences in T1w/T2w throughout the cerebral neocortex from childhood to early adulthood. We apply Bayesian spline models and clustering analysis to demonstrate graded variation in age-dependent cortical T1w/T2w differences that are correlated with the sensorimotor-association (S-A) axis of cortical organization reported by others. In sensorimotor areas, T1w/T2w ratio measures start at high levels at early ages, increase at a fast pace, and decelerate at later ages (18-21). In intermediate multimodal areas along the S-A axis, T1w/T2w starts at intermediate levels and increases linearly at an intermediate pace. In transmodal/paralimbic association areas, T1w/T2w starts at low levels and increases linearly at the slowest pace. These data provide evidence for graded variation of the T1w/T2w ratio along the S-A axis that may reflect cortical myelination changes during adolescence underlying the development of complex information processing and psychological functioning. We discuss the implications of these results as well as caveats in interpreting magnetic resonance imaging (MRI)-based estimates of myelination.SIGNIFICANCE STATEMENT Myelin is a lipid membrane that is essential to healthy brain function. Myelin wraps axons to increase neural signaling speed, enabling complex neuronal functioning underlying learning and cognition. Here, we characterize the developmental timing of myelination across the cerebral cortex during adolescence using a noninvasive proxy measure, T1w/T2w mapping. Our results provide new evidence demonstrating graded variation across the cortex in the timing of T1w/T2w changes during adolescence, with rapid T1w/T2w increases in lower-order sensory areas and gradual T1w/T2w increases in higher-order association areas. This spatial pattern of microstructural brain development closely parallels the sensorimotor-to-association axis of cortical organization and plasticity during ontogeny.
Keywords: MRI; T1w/T2w; adolescence; development; myelin.
Copyright © 2022 the authors.
Figures





References
-
- Baum GL, Roalf DR, Cook PA, Ciric R, Rosen AFG, Xia C, Elliott MA, Ruparel K, Verma R, Tunç B, Gur RC, Gur RE, Bassett DS, Satterthwaite TD (2018) The impact of in-scanner head motion on structural connectivity derived from diffusion MRI. Neuroimage 173:275–286. 10.1016/j.neuroimage.2018.02.041 - DOI - PMC - PubMed
-
- Bouveyron C, Jacques J (2011) Model-based clustering of time series in group-specific functional subspaces. Adv Data Anal Classif 5:281–300. 10.1007/s11634-011-0095-6 - DOI
-
- Bürkner P-C (2018) Advanced Bayesian multilevel modeling with the R package brms. R J 10:395–411. 10.32614/RJ-2018-017 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
