Membrane Stretch Gates NMDA Receptors
- PMID: 35705487
- PMCID: PMC9302457
- DOI: 10.1523/JNEUROSCI.0350-22.2022
Membrane Stretch Gates NMDA Receptors
Abstract
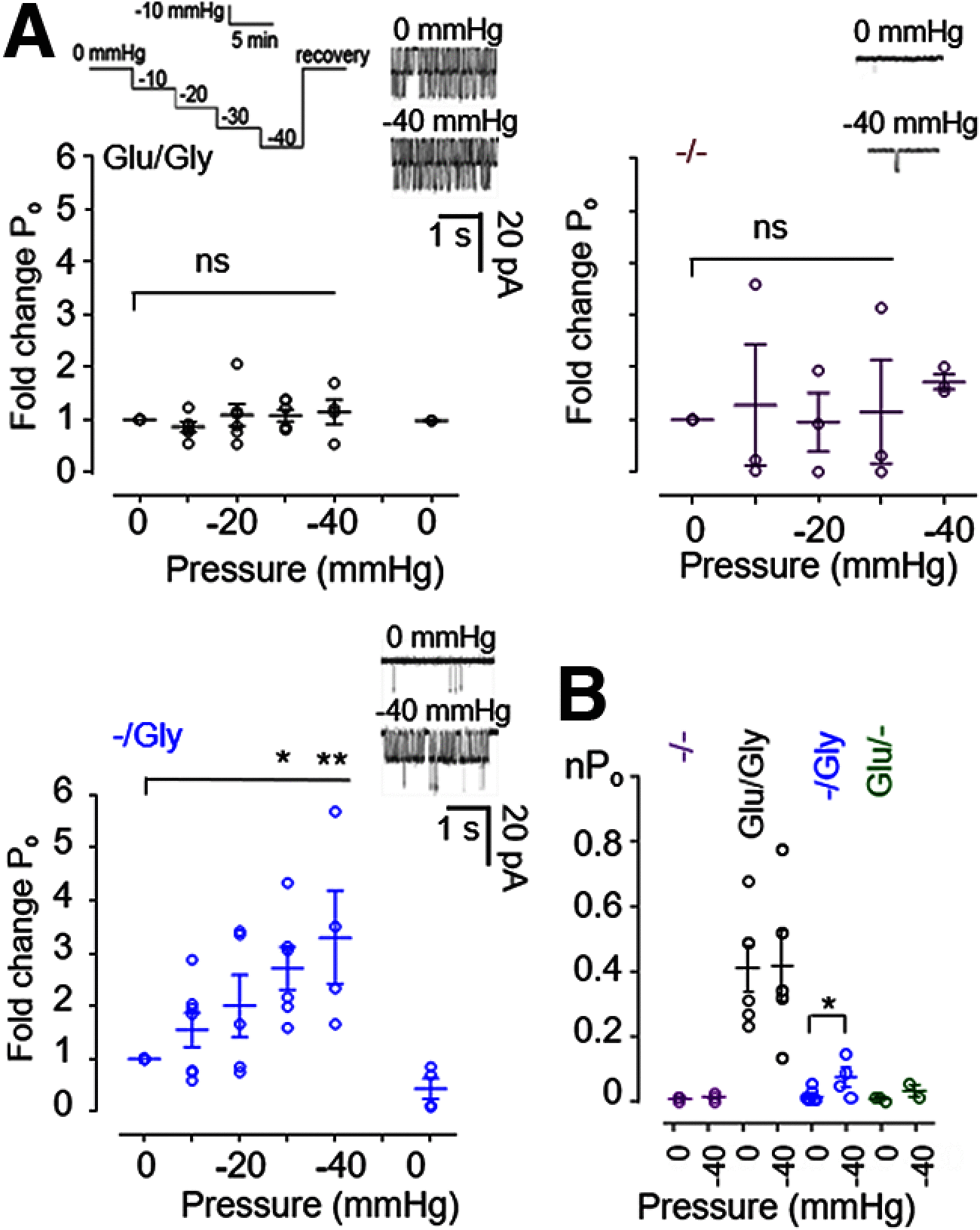
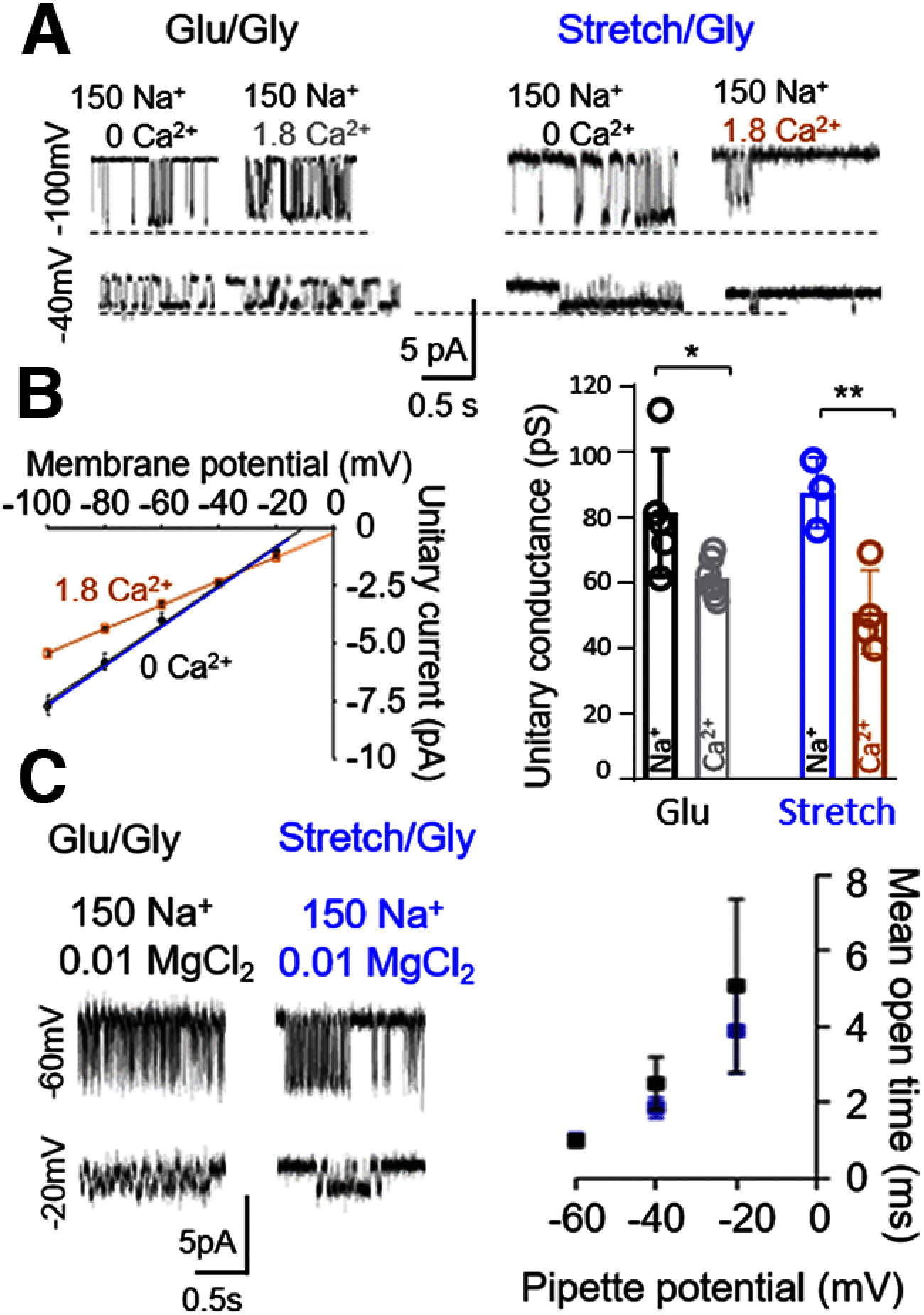
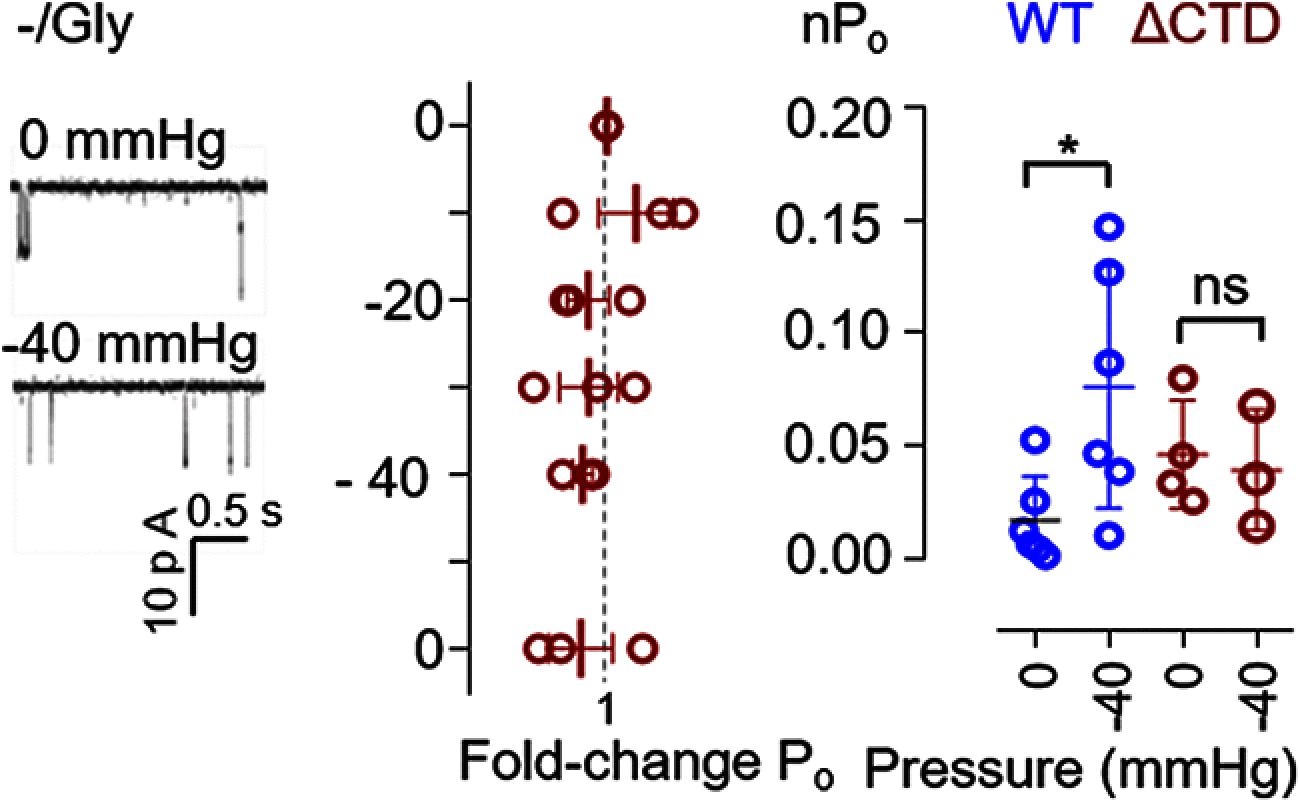
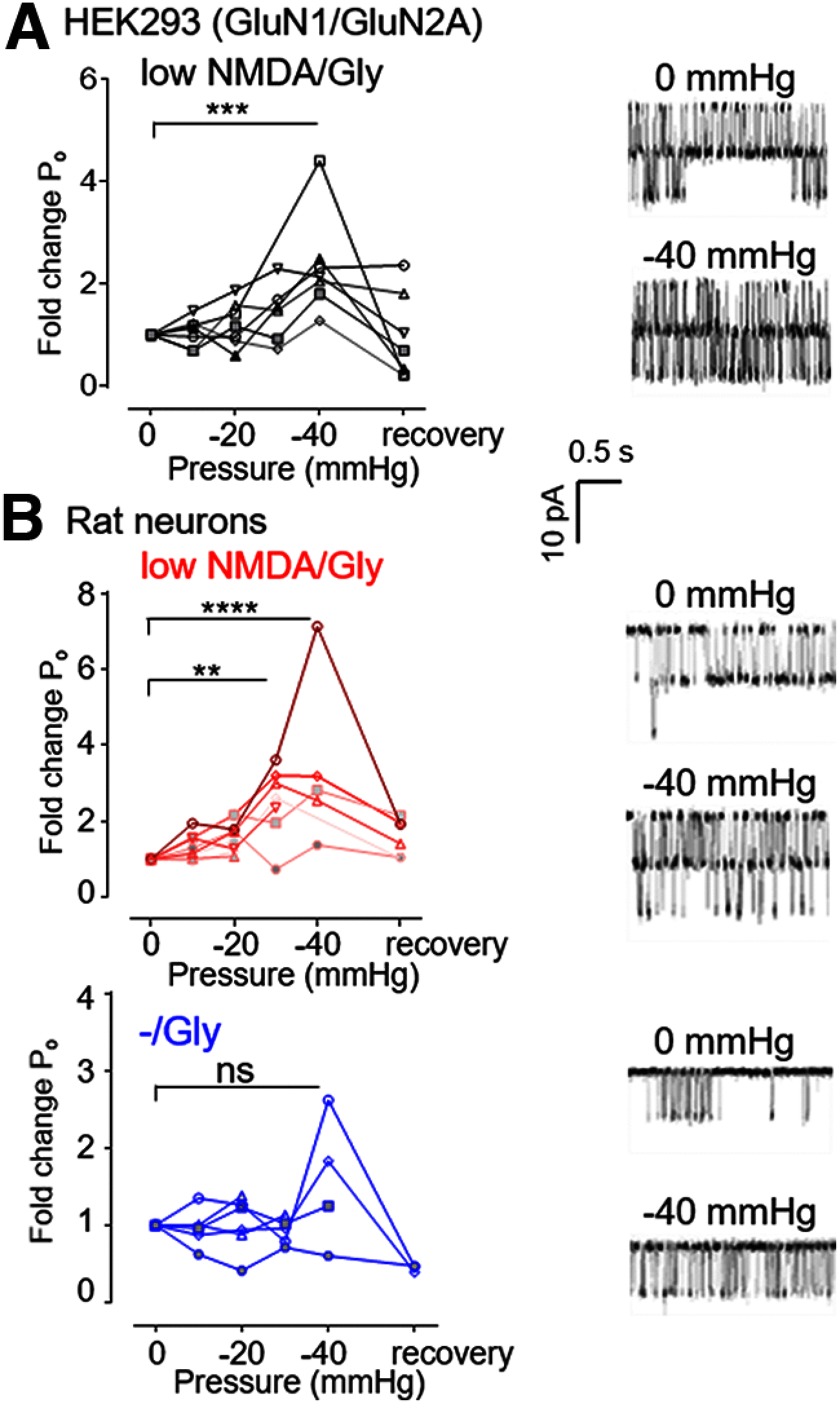
NMDARs are ionotropic glutamate receptors widely expressed in the CNS, where they mediate phenomena as diverse as neurotransmission, information processing, synaptogenesis, and cellular toxicity. They function as glutamate-gated Ca2+-permeable channels, which require glycine as coagonist, and can be modulated by many diffusible ligands and cellular cues, including mechanical stimuli. Previously, we found that, in cultured astrocytes, shear stress initiates NMDAR-mediated Ca2+ entry in the absence of added agonists, suggesting that more than being mechanosensitive, NMDARs may be mechanically activated. Here, we used controlled expression of rat recombinant receptors and noninvasive on-cell single-channel current recordings to show that mild membrane stretch can substitute for the neurotransmitter glutamate in gating NMDAR currents. Notably, stretch-activated currents maintained the hallmark features of the glutamate-gated currents, including glycine-requirement, large unitary conductance, high Ca2+ permeability, and voltage-dependent Mg2+ blockade. Further, we found that the stretch-gated current required the receptor's intracellular domain. Our results are consistent with the hypothesis that mechanical forces can gate endogenous NMDAR currents even in the absence of synaptic glutamate release, which has important implications for understanding mechanotransduction and the physiological and pathologic effects of mechanical forces on cells of the CNS.SIGNIFICANCE STATEMENT We show that, in addition to enhancing currents elicited with low agonist concentrations, membrane stretch can gate NMDARs in the absence of the neurotransmitter glutamate. Stretch-gated currents have the principal hallmarks of the glutamate-gated currents, including requirement for glycine, large Na+ conductance, high Ca2+ permeability, and voltage-dependent Mg2+ block. Therefore, results suggest that mechanical forces can initiate cellular processes presently attributed to glutamatergic neurotransmission, such as synaptic plasticity and cytotoxicity. Given the ubiquitous presence of mechanical forces in the CNS, this discovery identifies NMDARs as possibly important mechanotransducers during development and across the lifespan, and during pathologic processes, such as those associated with traumatic brain injuries, shaken infant syndrome, and chronic traumatic encephalopathy.
Keywords: NMDARs; ionotropic glutamate receptors; mechanotransduction; patch-clamp; signal transduction; single-molecule.
Copyright © 2022 the authors.
Figures
Comment in
-
Glycine-bound NMDA receptors are stretch-activated.Trends Neurosci. 2022 Nov;45(11):794-795. doi: 10.1016/j.tins.2022.08.002. Epub 2022 Aug 18. Trends Neurosci. 2022. PMID: 35989128
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
