Influence of Reaction Conditions on Enzymatic Enantiopreference: the Curious Case of HEwT in the Synthesis of THF-Amine
- PMID: 35705492
- PMCID: PMC9400895
- DOI: 10.1002/cbic.202200335
Influence of Reaction Conditions on Enzymatic Enantiopreference: the Curious Case of HEwT in the Synthesis of THF-Amine
Abstract
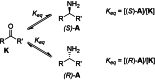
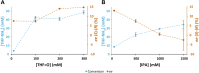
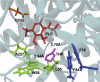
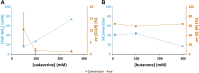
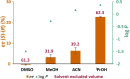
Enzymatic enantiopreference is one of the key advantages of biocatalysis. While exploring the synthesis of small cyclic (chiral amines) such as 3-aminotetrahydrofuran (THF-amine), using the (S)-selective transaminase from Halomonas elongata (HEwT), inversion of the enantiopreference was observed at increasing substrate loadings. In addition, the enantiopreference could be altered by variation of the ionic strength, or of the co-solvent content in the reaction mixture. For example, using otherwise identical reaction conditions, the presence of 2 M sodium chloride gave (R)-THF-amine (14 % ee), while the addition of 2.2 M isopropyl alcohol gave the (S)-enantiomer in 30 % ee. While the underlying cause is not currently understood, it appears likely that subtle changes in the structure of the enzyme cause the shift in enantiopreference and are worth exploring further.
Keywords: THF ketones; amines; biocatalysis; enantioselectivity; enzyme; transaminases.
© 2022 The Authors. ChemBioChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Hauer B., ACS Catal. 2020, 10, 8418–8427.
-
- Breuer M., Ditrich K., Habicher T., Hauer B., Keßeler M., Stürmer R., Zelinski T., Angew. Chem. Int. Ed. 2004, 43, 788–824; - PubMed
- Angew. Chem. 2004, 116, 806–843.
-
- Ghosh T., Ernst M., Hashmi A. S. K., Schaub T., Eur. J. Org. Chem. 2020, 4796–4800.
-
- Patil M. D., Grogan G., Bommarius A., Yun H., ACS Catal. 2018, 8, 10985–11015.
-
- Ghislieri D., Turner N. J., Top. Catal. 2014, 57, 284–300.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

