Long-term Efficacy and Safety From an Open-Label Extension of Adjunctive Cenobamate in Patients With Uncontrolled Focal Seizures
- PMID: 35705501
- PMCID: PMC9519254
- DOI: 10.1212/WNL.0000000000200792
Long-term Efficacy and Safety From an Open-Label Extension of Adjunctive Cenobamate in Patients With Uncontrolled Focal Seizures
Abstract
Background and objectives: To evaluate long-term efficacy (percent seizure frequency reduction and responder rates), safety, and tolerability of adjunctive cenobamate (CNB) in an open-label extension (OLE) of the randomized, double-blind, placebo-controlled study.
Methods: Patients (aged 18-70 years) with uncontrolled focal seizures despite treatment with 1-3 antiseizure medications who completed the 18-week double-blind study (n = 360) could enter the OLE, where they underwent a 2-week blinded conversion to CNB (target dose, 300 mg/d; min/max, 50/400 mg/d).
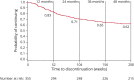
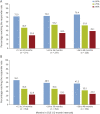
Results: Three hundred fifty-five patients were included in the OLE safety population (265 originally randomized to CNB, 90 originally randomized to placebo), and 354 were included in the OLE modified intent-to-treat population. As of July 2019, 58.9% of patients (209/355) were continuing CNB treatment and 141 had discontinued, including 16.6% (59/355) because of lack of efficacy, 8.7% (31/355) because of withdrawal by patient, and 7.6% (27/355) because of adverse events. The median (range) duration of OLE exposure was 53.9 (1.1-68.7) months. Retention rates at 12, 24, 36, and 48 months were 83%, 71%, 65%, and 62%, respectively. Median percent seizure frequency reduction over baseline increased with each 6-month OLE interval, up to 76.1% at months 43-48. Among observed patients, 16.4% (36/220) achieved 100% and 39.1% (86/220) achieved ≥90% seizure reduction during >36-48 months. Among the initial OLE modified intent-to-treat population, 10.2% of patients (36/354) achieved 100% and 24.3% (86/354) achieved ≥90% seizure reduction during >36-48 months. Similar to the double-blind study, adverse events (AEs) included dizziness, somnolence, fatigue, and headache. Serious AEs occurred in 20.3% of patients (72/355).
Discussion: Long-term efficacy, including 100% and ≥90% seizure reduction, was sustained during 48 months of CNB treatment, with 71% retention at 24 months. No new safety issues were identified. These results confirm the findings of the double-blind study and support the potential long-term clinical benefit of CNB.
Classification of evidence: This study provides Class IV evidence that oral CNB 50-400 mg/d is effective as an adjunctive treatment for the long-term management of patients with uncontrolled focal seizures previously treated with 1-3 ASMs.
Trial registration information: ClinicalTrials.gov NCT01866111 (clinicaltrials.gov/ct2/show/results/NCT01866111).
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
