Human seasonal coronavirus neutralization and COVID-19 severity
- PMID: 35705514
- PMCID: PMC9349487
- DOI: 10.1002/jmv.27937
Human seasonal coronavirus neutralization and COVID-19 severity
Abstract
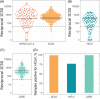
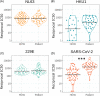
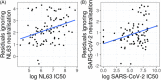
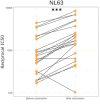
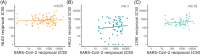
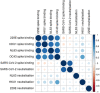
The virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for the global coronavirus disease-2019 (COVID-19) pandemic, spread rapidly around the world causing high morbidity and mortality. However, there are four known, endemic seasonal coronaviruses in humans (HCoVs), and whether antibodies for these HCoVs play a role in severity of COVID-19 disease has generated a lot of interest. Of these seasonal viruses NL63 is of particular interest as it uses the same cell entry receptor as SARS-CoV-2. We use functional, neutralizing assays to investigate cross-reactive antibodies and their relationship with COVID-19 severity. We analyzed the neutralization of SARS-CoV-2, NL63, HKU1, and 229E in 38 COVID-19 patients and 62 healthcare workers, and a further 182 samples to specifically study the relationship between SARS-CoV-2 and NL63. We found that although HCoV neutralization was very common there was little evidence that these antibodies neutralized SARS-CoV-2. Despite no evidence in cross-neutralization, levels of NL63 neutralizing antibodies become elevated after exposure to SARS-CoV-2 through infection or following vaccination.
Keywords: SARS coronavirus; endemic infection; epidemiology; immune responses; neutralization; virus classification.
© 2022 The Authors. Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
David A. Wells was employed to DIOSynVax at the time of this study. Matteo Ferrari, and Jonathan Heeney are currently employed/affiliated to DIOSynVax company. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. DIOSynVax did not provide any funding toward this study.
Figures


Similar articles
-
Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination Boosts Neutralizing Activity Against Seasonal Human Coronaviruses.Clin Infect Dis. 2022 Aug 24;75(1):e653-e661. doi: 10.1093/cid/ciac057. Clin Infect Dis. 2022. PMID: 35079775 Free PMC article.
-
Coronavirus Pseudotypes for All Circulating Human Coronaviruses for Quantification of Cross-Neutralizing Antibody Responses.Viruses. 2021 Aug 10;13(8):1579. doi: 10.3390/v13081579. Viruses. 2021. PMID: 34452443 Free PMC article.
-
Are higher antibody levels against seasonal human coronaviruses associated with a more robust humoral immune response after SARS-CoV-2 vaccination?Front Immunol. 2022 Sep 8;13:954093. doi: 10.3389/fimmu.2022.954093. eCollection 2022. Front Immunol. 2022. PMID: 36159791 Free PMC article.
-
An overview on the seven pathogenic human coronaviruses.Rev Med Virol. 2022 Mar;32(2):e2282. doi: 10.1002/rmv.2282. Epub 2021 Aug 2. Rev Med Virol. 2022. PMID: 34339073 Review.
-
Evolutionary trajectory of SARS-CoV-2 and emerging variants.Virol J. 2021 Aug 13;18(1):166. doi: 10.1186/s12985-021-01633-w. Virol J. 2021. PMID: 34389034 Free PMC article. Review.
Cited by
-
Comprehensive analysis of human coronavirus antibody responses in ICU and non-ICU COVID-19 patients reveals IgG3 against SARS-CoV-2 spike protein as a key biomarker of disease severity.J Med Microbiol. 2025 May;74(5):002012. doi: 10.1099/jmm.0.002012. J Med Microbiol. 2025. PMID: 40359129 Free PMC article.
-
SARS-CoV-2 mRNA Vaccines Induce Cross-Reactive Antibodies to NL63 Coronavirus but Do Not Boost Pre-Existing Immunity Anti-NL63 Antibody Responses.Vaccines (Basel). 2025 Mar 4;13(3):268. doi: 10.3390/vaccines13030268. Vaccines (Basel). 2025. PMID: 40266143 Free PMC article.
-
Analysis of Antibody Neutralisation Activity against SARS-CoV-2 Variants and Seasonal Human Coronaviruses NL63, HKU1, and 229E Induced by Three Different COVID-19 Vaccine Platforms.Vaccines (Basel). 2022 Dec 27;11(1):58. doi: 10.3390/vaccines11010058. Vaccines (Basel). 2022. PMID: 36679903 Free PMC article.
-
Conference Report: LPMHealthcare Emerging Viruses 2023 (EVOX23): Pandemics-Learning from the Past and Present to Prepare for the Future.Pathogens. 2024 Aug 10;13(8):679. doi: 10.3390/pathogens13080679. Pathogens. 2024. PMID: 39204279 Free PMC article.
-
Endemic Human Coronavirus Antibody Levels Are Unchanged after Convalescent or Control Plasma Transfusion for Early Outpatient COVID-19 Treatment.mBio. 2023 Feb 28;14(1):e0328722. doi: 10.1128/mbio.03287-22. Epub 2023 Jan 10. mBio. 2023. PMID: 36625657 Free PMC article. Clinical Trial.
References
-
- Hoek LVD. Human coronaviruses: What do they cause? Antiviral Therapy [Internet].2007. Accessed August 15, 2021. https://covid19.elsevierpure.com/en/publications/human-coronaviruses-wha...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

