Complete biosynthetic pathway to the antidiabetic drug acarbose
- PMID: 35705566
- PMCID: PMC9200736
- DOI: 10.1038/s41467-022-31232-4
Complete biosynthetic pathway to the antidiabetic drug acarbose
Abstract
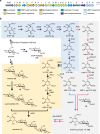
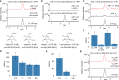
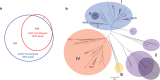
Acarbose is a bacterial-derived α-glucosidase inhibitor clinically used to treat patients with type 2 diabetes. As type 2 diabetes is on the rise worldwide, the market demand for acarbose has also increased. Despite its significant therapeutic importance, how it is made in nature is not completely understood. Here, we report the complete biosynthetic pathway to acarbose and its structural components, GDP-valienol and O-4-amino-(4,6-dideoxy-α-D-glucopyranosyl)-(1→4)-O-α-D-glucopyranosyl-(1→4)-D-glucopyranose. GDP-valienol is derived from valienol 7-phosphate, catalyzed by three cyclitol modifying enzymes, whereas O-4-amino-(4,6-dideoxy-α-D-glucopyranosyl)-(1→4)-O-α-D-glucopyranosyl-(1→4)-D-glucopyranose is produced from dTDP-4-amino-4,6-dideoxy-D-glucose and maltose by the glycosyltransferase AcbI. The final assembly process is catalyzed by a pseudoglycosyltransferase enzyme, AcbS, which is a homologue of AcbI but catalyzes the formation of a non-glycosidic C-N bond. This study clarifies all previously unknown steps in acarbose biosynthesis and establishes a complete pathway to this high value pharmaceutical.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Laube H, Fouladfar M, Aubell R, Schmitz H. [Effect of glucosidase inhibitor, Bay g 5421 (acarbose), on the blood glucose in obese diabetic patients ty pe 2 (NIDDM) (author’s transl)] Arzneimittelforschung. 1980;30:1154–1157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

