Decoronation-induced infected alveolar socket defect rat model for ridge preservation
- PMID: 35705614
- PMCID: PMC9200756
- DOI: 10.1038/s41598-022-14064-6
Decoronation-induced infected alveolar socket defect rat model for ridge preservation
Abstract
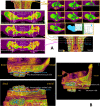
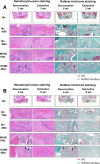
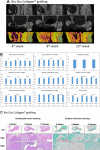
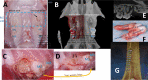
Current rat alveolar ridge preservation models have not been well standardized. In this study, we proposed decoronation-induced infected alveolar socket model of rat. The bilateral maxillary first molars (M1) of twenty-four rats were decoronized or extracted. After 2, 6, 10, and 14 weeks, bone and soft tissue changes at M1 and periodontal conditions of maxillary second (M2) and third molars (M3) were evaluated by micro-computed tomography and histological analysis. Additional eighteen rats with standardized size defects were grafted with Bio-Oss Collagen to compare with unmanipulated contralateral side. Decoronation preserved greater bone and soft tissue dimensions at M1, provided larger three-dimensional (3D) bone contour volume, but also promoted periodontal breakdown of M2 Histological results showed intense inflammatory cell infiltrations and severe bone resorption within M1 socket and at mesial aspect of M2. The critical dimensions to accommodate largest standardized defect at M1 were 2.2-2.3 mm at vertical bone height and 2.8-3.2 mm at alveolar crestal width. Bio-Oss Collagen could not fully preserve buccal or palatal bone height but could be beneficial in preserving ridge width in large alveolar defects. Collectively, if periodontally-involved alveolar bone defect is preferred, we suggest extracting M1 roots 6 weeks after decoronation to allow periodontitis to occur at M2. If standardized critical dimension defect is preferred, we suggest extracting M1 roots 2 weeks after decoronation, and creating defect in the middle of M1 site with size no larger than 2.7 mm diameter to its full depth.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Schropp L, Wenzel A, Kostopoulos L, Karring T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003;23:313–323. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

