Validation of the forced swim test in Drosophila, and its use to demonstrate psilocybin has long-lasting antidepressant-like effects in flies
- PMID: 35705666
- PMCID: PMC9200711
- DOI: 10.1038/s41598-022-14165-2
Validation of the forced swim test in Drosophila, and its use to demonstrate psilocybin has long-lasting antidepressant-like effects in flies
Abstract
Psilocybin has been shown to be a powerful, long-lasting antidepressant in human clinical trials and in rodent models. Although rodents have commonly been used to model psychiatric disorders, Drosophila have neurotransmitter systems similar to mammals and many comparable brain structures involved in similar behaviors. The forced swim test (FST), which has been used extensively to evaluate compounds for antidepressant efficacy, has recently been adapted for Drosophila. The fly FST has potential to be a cost-effective, high-throughput assay for evaluating potential antidepressants. For this study we pharmacologically validated the fly FST using methamphetamine, DL-α-methyltyrosine, and the antidepressant citalopram. While methamphetamine and DL-α-methyltyrosine altered overall locomotor activity in the Drosophila Activity Monitor System (DAMS), they had no significant impact on measures of immobility in the FST. Conversely, chronic citalopram decreased measures of immobility in the FST in both sexes without increasing DAMS activity. We used the validated FST to evaluate the antidepressant-like effects of high (3.5 mM) and low (0.03 mM) doses of psilocybin. Both doses of psilocybin significantly reduced measures of immobility in male flies, but not females. 0.03 mM had an effect size comparable to chronic citalopram, and 3.5 mM had an effect size approximately twice that of chronic citalopram.
© 2022. The Author(s).
Conflict of interest statement
M.H. has no competing interests. C.D. Nichols is a member of the Scientific Advisory Board of Eleusis Therapeutics, and has a Sponsored Research Contract with Eleusis Therapeutics.
Figures




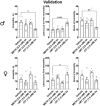
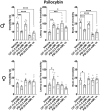
References
-
- O’Kane, C. J. Drosophila as a model organism for the study of neuropsychiatric disorders. in Molecular and Functional Models in Neuropsychiatry. 37–60. 10.1007/7854_2010_110 (Springer, 2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

