An exercise-inducible metabolite that suppresses feeding and obesity
- PMID: 35705806
- PMCID: PMC9767481
- DOI: 10.1038/s41586-022-04828-5
An exercise-inducible metabolite that suppresses feeding and obesity
Abstract
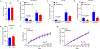
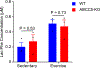
Exercise confers protection against obesity, type 2 diabetes and other cardiometabolic diseases1-5. However, the molecular and cellular mechanisms that mediate the metabolic benefits of physical activity remain unclear6. Here we show that exercise stimulates the production of N-lactoyl-phenylalanine (Lac-Phe), a blood-borne signalling metabolite that suppresses feeding and obesity. The biosynthesis of Lac-Phe from lactate and phenylalanine occurs in CNDP2+ cells, including macrophages, monocytes and other immune and epithelial cells localized to diverse organs. In diet-induced obese mice, pharmacological-mediated increases in Lac-Phe reduces food intake without affecting movement or energy expenditure. Chronic administration of Lac-Phe decreases adiposity and body weight and improves glucose homeostasis. Conversely, genetic ablation of Lac-Phe biosynthesis in mice increases food intake and obesity following exercise training. Last, large activity-inducible increases in circulating Lac-Phe are also observed in humans and racehorses, establishing this metabolite as a molecular effector associated with physical activity across multiple activity modalities and mammalian species. These data define a conserved exercise-inducible metabolite that controls food intake and influences systemic energy balance.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interest declaration
The authors declare the following competing interests: a provisional patent application has been filed by Stanford University on lactoyl amino acids for the treatment of metabolic disease.
Figures

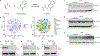
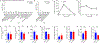




Comment in
-
Exercise molecule burns away hunger.Nature. 2022 Jun;606(7915):655-656. doi: 10.1038/d41586-022-01321-x. Nature. 2022. PMID: 35705863 No abstract available.
-
Mimicking the beneficial effects of exercise.Nat Rev Drug Discov. 2022 Aug;21(8):555. doi: 10.1038/d41573-022-00110-5. Nat Rev Drug Discov. 2022. PMID: 35764829 No abstract available.
-
An exercise-induced metabolite alters feeding.Nat Rev Endocrinol. 2022 Sep;18(9):521. doi: 10.1038/s41574-022-00719-x. Nat Rev Endocrinol. 2022. PMID: 35790834 No abstract available.
References
-
- Eriksson KF & Lindgärde F Prevention of Type 2 (non-insulin-dependent) diabetes mellitus by diet and physical exercise. Diabetologia 34, 891–898 (1991). - PubMed
-
- Stampfer MJ, Hu FB, Manson JE, Rimm EB & Willett WC Primary prevention of coronary heart disease in women through diet and lifestyle. N. Engl. J. Med. 343, 16–22 (2000). - PubMed
-
- Helmrich SP, Ragland DR, Leung RW & Paffenbarger RS Physical activity and reduced occurrence of non-insulin-dependent diabetes mellitus. New English J. Med. 325, 147–152 (1991). - PubMed
-
- Rawshani A et al. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 379, 633–644 (2018). - PubMed
Methods References
-
- De Wolf CJF et al. cGMP transport by vesicles from human and mouse erythrocytes. FEBS J. 274, 439–450 (2007). - PubMed
-
- Smith CA et al. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78, 779–787 (2006). - PubMed
-
- Agudelo LZ et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159, 33–45 (2014). - PubMed
MeSH terms
Substances
Grants and funding
- DK130541/NH/NIH HHS/United States
- R01 DK124265/DK/NIDDK NIH HHS/United States
- P01 DK113954/DK/NIDDK NIH HHS/United States
- R01 DK120858/DK/NIDDK NIH HHS/United States
- R01 DK118940/DK/NIDDK NIH HHS/United States
- T32 GM113854/GM/NIGMS NIH HHS/United States
- R01 DK115761/DK/NIDDK NIH HHS/United States
- DP1 DK130641/DK/NIDDK NIH HHS/United States
- R01 DK117281/DK/NIDDK NIH HHS/United States
- 20POST35120600/AHA/American Heart Association-American Stroke Association/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- GM113854/NH/NIH HHS/United States
- DK113954/NH/NIH HHS/United States
- R01 AR072695/AR/NIAMS NIH HHS/United States
- R01 DK126830/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

