Superior control of inflammatory pain by corticotropin-releasing factor receptor 1 via opioid peptides in distinct pain-relevant brain areas
- PMID: 35705992
- PMCID: PMC9199204
- DOI: 10.1186/s12974-022-02498-8
Superior control of inflammatory pain by corticotropin-releasing factor receptor 1 via opioid peptides in distinct pain-relevant brain areas
Abstract
Background: Under inflammatory conditions, the activation of corticotropin-releasing factor (CRF) receptor has been shown to inhibit pain through opioid peptide release from immune cells or neurons. CRF's effects on human and animal pain modulation depend, however, on the distribution of its receptor subtypes 1 and 2 (CRF-R1 and CRF-R2) along the neuraxis of pain transmission. The objective of this study is to investigate the respective role of each CRF receptor subtype on centrally administered CRF-induced antinociception during inflammatory pain.
Methods: The present study investigated the role of intracerebroventricular (i.c.v.) CRF receptor agonists on nociception and the contribution of cerebral CRF-R1 and/or CRF-R2 subtypes in an animal model of Freund's complete adjuvant (FCA)-induced hind paw inflammation. Methods used included behavioral experiments, immunofluorescence confocal analysis, and reverse transcriptase-polymerase chain reaction.
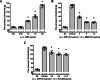
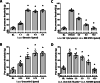
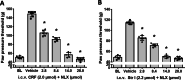
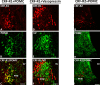
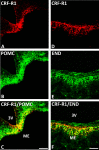
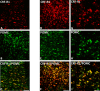
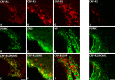
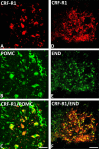
Results: Intracerebroventricular, but systemically inactive, doses of CRF elicited potent, dose-dependent antinociceptive effects in inflammatory pain which were significantly antagonized by i.c.v. CRF-R1-selective antagonist NBI 27914 (by approximately 60%) but less by CRF-R2-selective antagonist K41498 (by only 20%). In line with these findings, i.c.v. administration of CRF-R1 agonist stressin I produced superior control of inflammatory pain over CRF-R2 agonist urocortin-2. Intriguingly, i.c.v. opioid antagonist naloxone significantly reversed the CRF as well as CRF-R1 agonist-elicited pain inhibition. Consistent with existing evidence of high CRF concentrations in brain areas such as the thalamus, hypothalamus, locus coeruleus, and periaqueductal gray following its i.c.v. administration, double-immunofluorescence confocal microscopy demonstrated primarily CRF-R1-positive neurons that expressed opioid peptides in these pain-relevant brain areas. Finally, PCR analysis confirmed the predominant expression of the CRF-R1 over CRF-R2 in representative brain areas such as the hypothalamus.
Conclusion: Taken together, these findings suggest that CRF-R1 in opioid-peptide-containing brain areas plays an important role in the modulation of inflammatory pain and may be a useful therapeutic target for inflammatory pain control.
Keywords: Brain; Corticotropin-releasing factor; Immunofluorescence; Inflammatory pain; Opioid peptide.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

