Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): a randomized, double blind, placebo controlled, single center, phase 2 study
- PMID: 35706006
- PMCID: PMC9198622
- DOI: 10.1186/s12967-022-03466-9
Rationale and design of interleukin-1 blockade in recently decompensated heart failure (REDHART2): a randomized, double blind, placebo controlled, single center, phase 2 study
Abstract
Background: Heart failure (HF) is a global leading cause of mortality despite implementation of guideline directed therapy which warrants a need for novel treatment strategies. Proof-of-concept clinical trials of anakinra, a recombinant human Interleukin-1 (IL-1) receptor antagonist, have shown promising results in patients with HF.
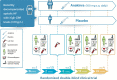
Method: We designed a single center, randomized, placebo controlled, double-blind phase II randomized clinical trial. One hundred and two adult patients hospitalized within 2 weeks of discharge due to acute decompensated HF with reduced ejection fraction (HFrEF) and systemic inflammation (high sensitivity of C-reactive protein > 2 mg/L) will be randomized in 2:1 ratio to receive anakinra or placebo for 24 weeks. The primary objective is to determine the effect of anakinra on peak oxygen consumption (VO2) measured at cardiopulmonary exercise testing (CPX) after 24 weeks of treatment, with placebo-corrected changes in peak VO2 at CPX after 24 weeks (or longest available follow up). Secondary exploratory endpoints will assess the effects of anakinra on additional CPX parameters, structural and functional echocardiographic data, noninvasive hemodynamic, quality of life questionnaires, biomarkers, and HF outcomes.
Discussion: The current trial will assess the effects of IL-1 blockade with anakinra for 24 weeks on cardiorespiratory fitness in patients with recent hospitalization due to acute decompensated HFrEF.
Trial registration: The trial was registered prospectively with ClinicalTrials.gov on Jan 8, 2019, identifier NCT03797001.
Keywords: Anakinra; HFrEF; Heart failure; IL-1; Interleukin-1; Target therapy; Treatment.
© 2022. The Author(s).
Conflict of interest statement
Dr. Abbate (AA) has served as consultant to Swedish Orphan Biovitrum in the past. Dr. Van Tassell (BVT) has served as consultant to Swedish Orphan Biovitrum in the past. No other authors have relevant disclosures.
Figures
References
-
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–e596. - PubMed
-
- Rauchhaus M, Doehner W, Francis DP, Davos C, Kemp M, Liebenthal C, et al. Plasma cytokine parameters and mortality in patients with chronic heart failure. Circulation. 2000;102(25):3060–3067. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

