Microglial amyloid beta clearance is driven by PIEZO1 channels
- PMID: 35706029
- PMCID: PMC9199162
- DOI: 10.1186/s12974-022-02486-y
Microglial amyloid beta clearance is driven by PIEZO1 channels
Abstract
Background: Microglia are the endogenous immune cells of the brain and act as sensors of pathology to maintain brain homeostasis and eliminate potential threats. In Alzheimer's disease (AD), toxic amyloid beta (Aβ) accumulates in the brain and forms stiff plaques. In late-onset AD accounting for 95% of all cases, this is thought to be due to reduced clearance of Aβ. Human genome-wide association studies and animal models suggest that reduced clearance results from aberrant function of microglia. While the impact of neurochemical pathways on microglia had been broadly studied, mechanical receptors regulating microglial functions remain largely unexplored.
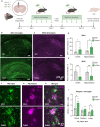
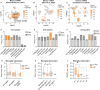
Methods: Here we showed that a mechanotransduction ion channel, PIEZO1, is expressed and functional in human and mouse microglia. We used a small molecule agonist, Yoda1, to study how activation of PIEZO1 affects AD-related functions in human induced pluripotent stem cell (iPSC)-derived microglia-like cells (iMGL) under controlled laboratory experiments. Cell survival, metabolism, phagocytosis and lysosomal activity were assessed using real-time functional assays. To evaluate the effect of activation of PIEZO1 in vivo, 5-month-old 5xFAD male mice were infused daily with Yoda1 for two weeks through intracranial cannulas. Microglial Iba1 expression and Aβ pathology were quantified with immunohistochemistry and confocal microscopy. Published human and mouse AD datasets were used for in-depth analysis of PIEZO1 gene expression and related pathways in microglial subpopulations.
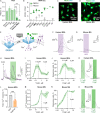
Results: We show that PIEZO1 orchestrates Aβ clearance by enhancing microglial survival, phagocytosis, and lysosomal activity. Aβ inhibited PIEZO1-mediated calcium transients, whereas activation of PIEZO1 with a selective agonist, Yoda1, improved microglial phagocytosis resulting in Aβ clearance both in human and mouse models of AD. Moreover, PIEZO1 expression was associated with a unique microglial transcriptional phenotype in AD as indicated by assessment of cellular metabolism, and human and mouse single-cell datasets.
Conclusion: These results indicate that the compromised function of microglia in AD could be improved by controlled activation of PIEZO1 channels resulting in alleviated Aβ burden. Pharmacological regulation of these mechanoreceptors in microglia could represent a novel therapeutic paradigm for AD.
Keywords: Alzheimer’s disease; Amyloid; Mechanoreceptor; Microglia; Piezo1; iMGL; iPSC-derived microglia.
© 2022. The Author(s).
Conflict of interest statement
Malm and Giniatullin are inventors of PCT patent application related to this topic.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

