A gossypol derivative effectively protects against Zika and dengue virus infection without toxicity
- PMID: 35706035
- PMCID: PMC9202104
- DOI: 10.1186/s12915-022-01344-w
A gossypol derivative effectively protects against Zika and dengue virus infection without toxicity
Abstract
Background: Zika virus (ZIKV) and dengue virus (DENV) cause microcephaly and dengue hemorrhagic fever, respectively, leading to severe problems. No effective antiviral agents are approved against infections of these flaviviruses, calling for the need to develop potent therapeutics. We previously identified gossypol as an effective inhibitor against ZIKV and DENV infections, but this compound is toxic and not suitable for in vivo treatment.
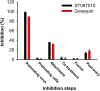
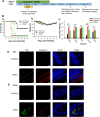
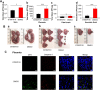
Results: In this study, we showed that gossypol derivative ST087010 exhibited potent and broad-spectrum in vitro inhibitory activity against infections of at least ten ZIKV strains isolated from different hosts, time periods, and countries, as well as DENV-1-4 serotypes, and significantly reduced cytotoxicity compared to gossypol. It presented broad-spectrum in vivo protective efficacy, protecting ZIKV-infected Ifnar1-/- mice from lethal challenge, with increased survival and reduced weight loss. Ifnar1-/- mice treated with this gossypol derivative decreased viral titers in various tissues, including the brain and testis, after infection with ZIKV at different human isolates. Moreover, ST087010 potently blocked ZIKV vertical transmission in pregnant Ifnar1-/- mice, preventing ZIKV-caused fetal death, and it was safe for pregnant mice and their pups. It also protected DENV-2-challenged Ifnar1-/- mice against viral replication by reducing the viral titers in the brain, kidney, heart, and sera.
Conclusions: Overall, our data indicate the potential for further development of this gossypol derivative as an effective and safe broad-spectrum therapeutic agent to treat ZIKV and DENV diseases.
Keywords: Antiviral agent; Dengue virus; Flavivirus; In vitro inhibition; In vivo protection; Toxicity; Zika virus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors.Viruses. 2019 Nov 2;11(11):1019. doi: 10.3390/v11111019. Viruses. 2019. PMID: 31684080 Free PMC article.
-
Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus.mBio. 2016 Jul 19;7(4):e01123-16. doi: 10.1128/mBio.01123-16. mBio. 2016. PMID: 27435464 Free PMC article.
-
Cross-Reactive T Cell Immunity to Dengue and Zika Viruses: New Insights Into Vaccine Development.Front Immunol. 2019 Jun 11;10:1316. doi: 10.3389/fimmu.2019.01316. eCollection 2019. Front Immunol. 2019. PMID: 31244855 Free PMC article. Review.
-
Antiviral activity of the natural alkaloid anisomycin against dengue and Zika viruses.Antiviral Res. 2020 Apr;176:104749. doi: 10.1016/j.antiviral.2020.104749. Epub 2020 Feb 17. Antiviral Res. 2020. PMID: 32081740 Free PMC article.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
Cited by
-
Envelope Protein-Targeting Zika Virus Entry Inhibitors.Int J Mol Sci. 2024 Aug 30;25(17):9424. doi: 10.3390/ijms25179424. Int J Mol Sci. 2024. PMID: 39273370 Free PMC article. Review.
-
Metabolic reprogramming in viral infections: the interplay of glucose metabolism and immune responses.Front Immunol. 2025 May 16;16:1578202. doi: 10.3389/fimmu.2025.1578202. eCollection 2025. Front Immunol. 2025. PMID: 40453076 Free PMC article. Review.
-
Therapeutic Use of Parerythrobacter sp. M20A3S10, a Marine Bacterium, Targeting Influenza Viruses and Flaviviruses.Animals (Basel). 2025 Jul 18;15(14):2125. doi: 10.3390/ani15142125. Animals (Basel). 2025. PMID: 40723588 Free PMC article.
-
An Overview of Zika Virus and Zika Virus Induced Neuropathies.Int J Mol Sci. 2024 Dec 24;26(1):47. doi: 10.3390/ijms26010047. Int J Mol Sci. 2024. PMID: 39795906 Free PMC article. Review.
-
Lactate facilitated mitochondrial fission-derived ROS to promote pulmonary fibrosis via ERK/DRP-1 signaling.J Transl Med. 2024 May 21;22(1):479. doi: 10.1186/s12967-024-05289-2. J Transl Med. 2024. PMID: 38773615 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

